Publications
Books

N-HETEROCYCLIC CARBENES: From Laboratory Curiosities to Efficient Synthetic Tools
Second Edition
Silvia Díez-González, Editor
In less than 20 years N-heterocyclic carbenes (NHCs) have become well-established ancillary ligands for the preparation of transition metal-based catalysts. This is mainly due to the fact that NHCs tend to bind strongly to metal centres, avoiding the need of excess ligand in catalytic reactions. Also, NHC‒metal complexes are often insensitive to air and moisture, and have proven remarkably resistant to oxidation. This book showcases the wide variety of applications of NHCs in different chemistry fields beyond being simple phosphine mimics. This second edition has been updated throughout, and now includes a new chapter on NHC‒main group element complexes. It covers the synthesis of NHC ligands and their corresponding metal complexes, as well as their bonding and stereoelectronic properties and applications in catalysis. This is complemented by related topics such as organocatalysis and biologically active complexes. Written for organic and inorganic chemists, this book is ideal for postgraduates, researchers and industrialists.
For the first edition of this volume, see: RSC Catalysis Series, 2010
Recent publications
57. Meyer-Schuster rearrangement of propargylic alcohols mediated by phosphorus-containing Brønsted acid catalysts
Radtanajiravong, L.; Peters, J.; Hummell, J.; Díez-González, S.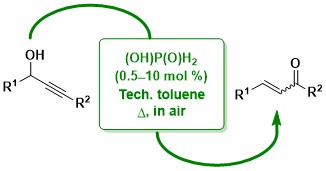
Org. Biomol. Chem. 2022, 20, 7338–7342
Commercially available (aqueous) hypophosphorus acid is an efficient catalyst for the synthesis of a,b-unsaturated carbonyl compounds from their corresponding propargylic alcohols. Reactions were carried out in technical toluene in the presence of air and in several instances the desired products were isolated analytically pure after a simple work-up.
56. Expedient metal-free preparation of aryl aziridines via thermal cycloaddition reactions
Sebest, F.; Radtanajiravong, L.; Kaukver, S.; White, A. J. P.; Díez-González, S.
Chem. Commun. 2022, 58, 3681–3184
A straightforward synthesis of aryl aziridines is reported from readily available azides and alkenes and using technical solvents in the presence of air. This methodology does not require any additives and the obtained compounds can be employed in ring opening and ring expansion reactions.
55. Cycloaddition reactions of azides and electron-deficient alkenes in deep eutectic solvents: Pyrazolines, aziridines and other surprises
Sebest, F.; Lachhani, K.; Pimpasri, C.; Casarrubios, L.; White, A. J. P.; Rzepa, H. S.; Díez-González, S.
Adv. Synth. Catal. 2020, 362, 1877–1886
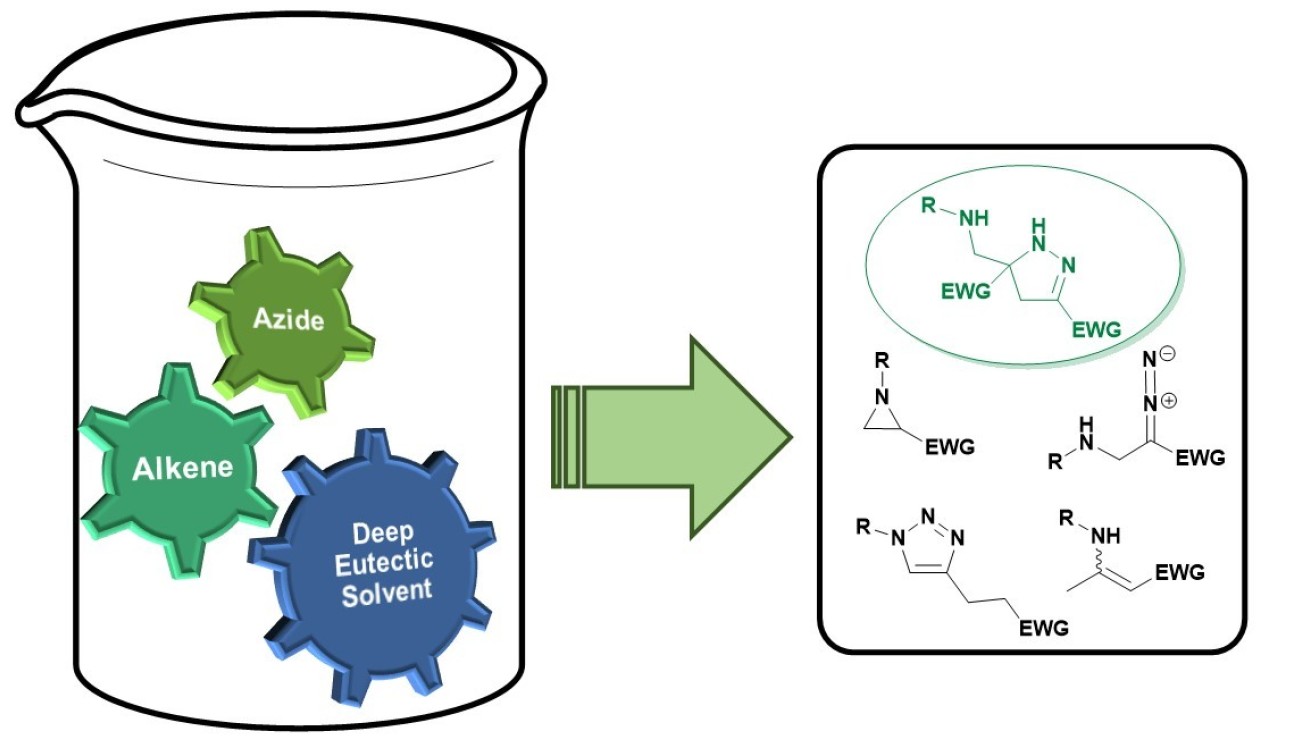
The reaction of organic azides and electron‐deficient alkenes was investigated in a deep eutectic solvents. A series of highly substituted 2‐pyrazolines was successfully isolated and their formation rationalised by DFT calculations. The critical effect of substitution was also explored; even relatively small changes in the cycloaddition partners led to completely different reaction outcomes and triazolines, triazoles or enaminones can be formed as major products depending on the alkene employed.
54. User-Friendly Copper-catalysed Reduction of Azides to Amines
Pimpasri, C.; White, A. J. P.; Díez-González, S.
Asian J. Org. Chem. 2020, 9,399-405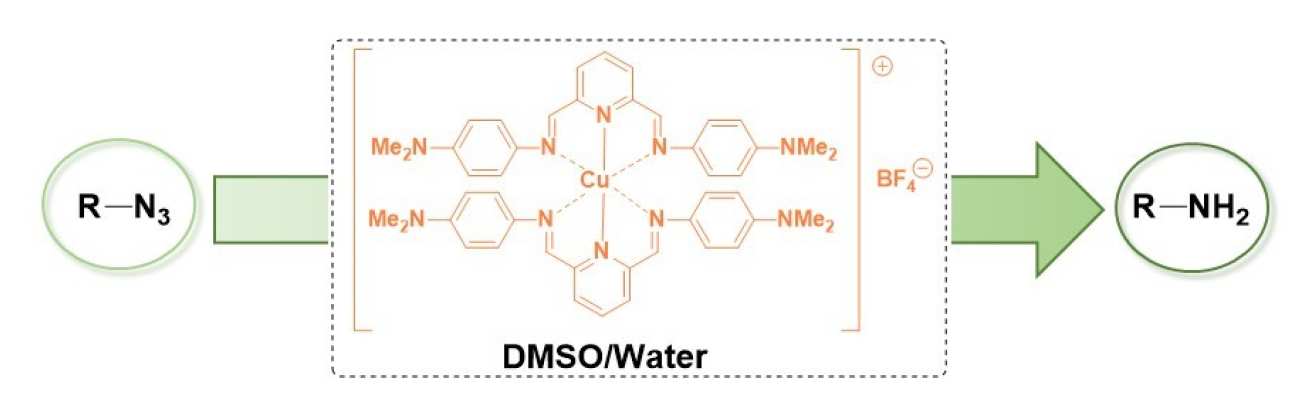
Three homoleptic copper(I) complexes have been prepared and applied to the reduction of organic azides. Under optimised conditions, complex [Cu(HPDIDMA)2]BF4 3 (HPDIDMA = 2,6-bis[N-(4-dimethylaminophenyl)carbaldimino]pyridine) could reduce a range of electron-poor azides in a mixture of DMSO and water without the need of an additional H-source.
53. Metal-Free 1,2,3-Triazole Synthesis in Deep Eutectic Solvents
Sebest, F.; Haselgrove, S.; White, A. J. P.; Díez-González, S.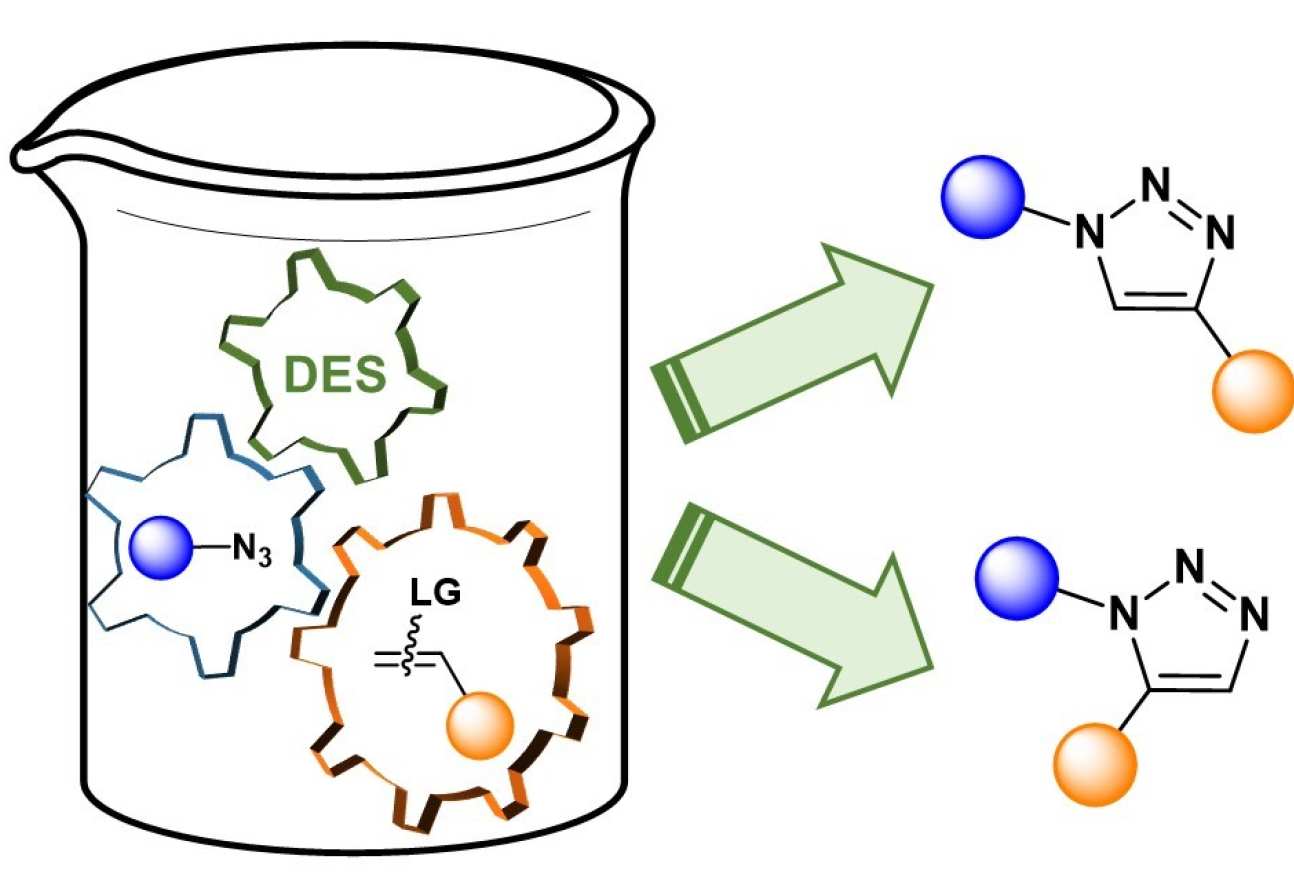
The metal-free regioselective preparation of 1,5- and 1,4-disubstituted triazoles is reported through a cycloaddition-elimination sequence. Reactions were carried out in environmentally friendly DES and pure products were isolated without the need of chromatographic techniques
52. Taming Brønsted Acid Reactivity: Nucleophilic Substitutions of Propargylic Alcohols with N-Nucleophiles Mediated by Phosphorus-Based Brønsted Acid Catalysts
Radtanajiravong, L.; Díez-González, S.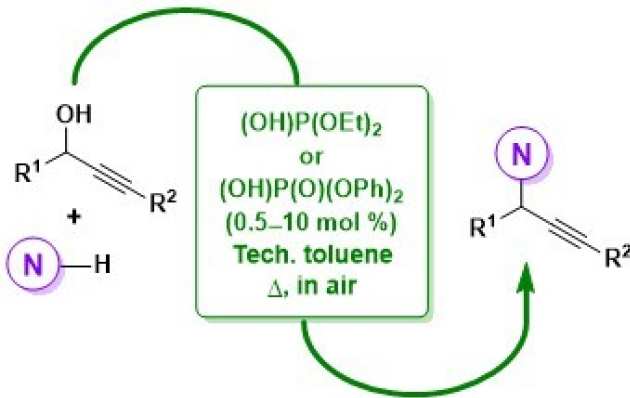
ACS Omega 2019, 4, 12300-12307.
The activity of diethyl phosphite and diphenyl phosphate in propargylation reactions with N-nucleophiles of varying basicity is presented. A careful choice of the reaction conditions minimized undesired rearrangements and arylation processes, typical side reactions with Brønsted acid catalysis. These systems are compatible with technical solvents and presence of air, and they are also applicable to C-, O-, and S-nucleophiles.
51. [Cu(NCMe)4](BF4): Commercially available and user-friendly catalyst for intramolecular hydroamination reactions
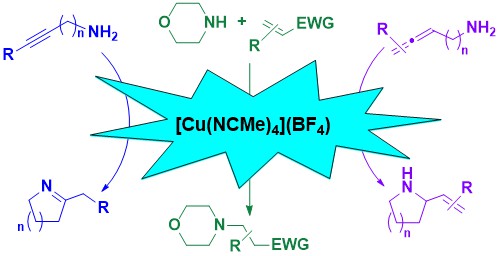
Zelenay, B.; Munton, P.; Tian, X.; Díez-González, S.
Eur. J. Org. Chem. 2019, 4, 4725–4730
The activity of a simple, commercially available copper salt, [Cu(NCMe)4](BF4) in intramolecular hydroamination reactions of alkynes and allenes is presented. Reactions were successfully carried out in technical acetonitrile in the presence of air. While attempts of alkene hydroamination failed, this catalysts was also found active in intermolecular aza-Michael reactions.
50. Cp*Fe(Me2PCH2CH2PMe2)(CHO): Hydride shuttle reactivity of a thermally stable formyl complex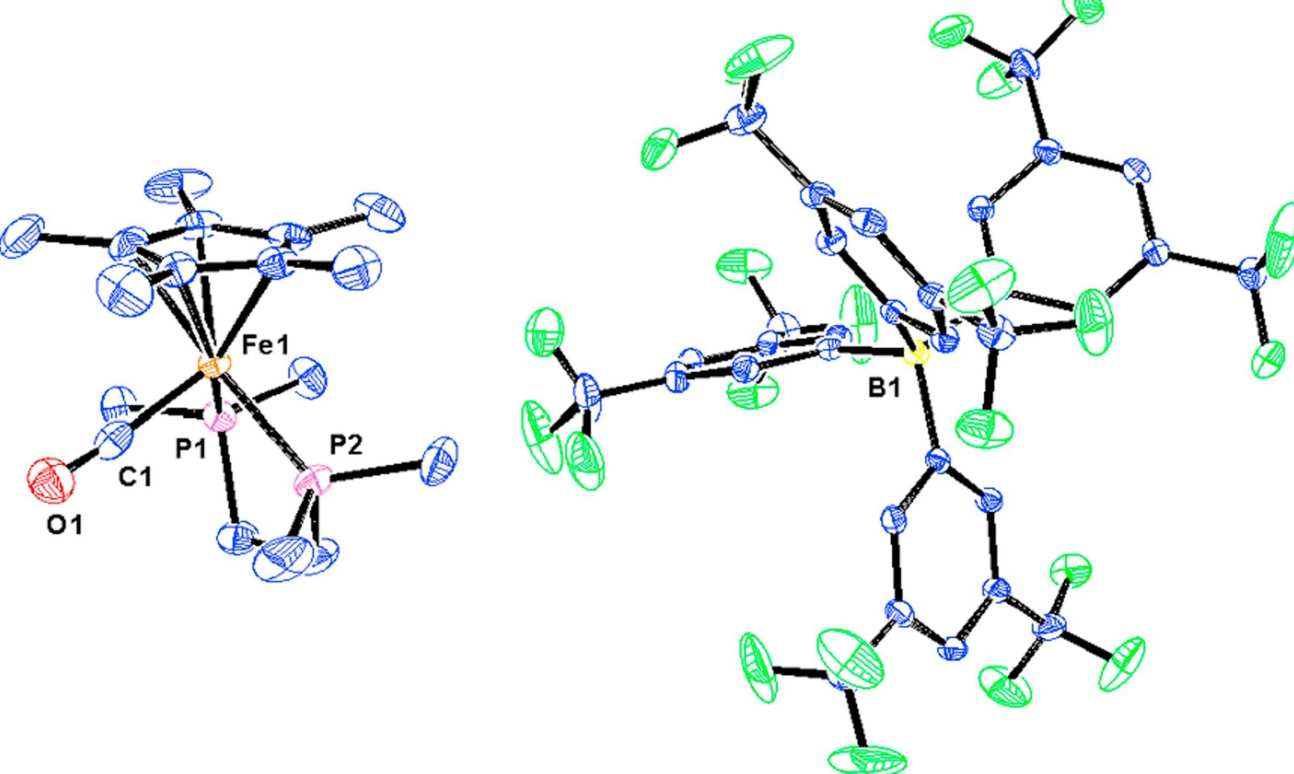
Sapsford, J. S.; Gates, S. J.; Doyle, L. R.; Taylor, R. A.; Díez-González, S.; Ashley, A. E.
Inorg. Chim. Acta 2019, 488, 201–207
[Cp*Fe(Me2PCH2CH2PMe2)(CO)]+ [BArF24]− has been synthesised and characterised using single crystal X-ray diffraction, NMR and IR spectroscopies. Reduction of the CO ligand using Na[Et3BH] produces the corresponding neutral formyl complex Cp*Fe(Me2PCH2CH2PMe2)(CHO), that is very thermally stable, and which is attributed to the electron-releasing properties of the spectator ligands. This compound is a potent hydride donor which exists in equilibrium with [Et3BH]−, Et3B, and the structural isomer (η4-C5Me5H)Cp*Fe(Me2PCH2CH2PMe2)(CO), resulting from reversible hydride migration to the Cp* ligand.
49. Copper-mediated reduction of azides under seemingly oxidising conditions: Catalytic and computational studies
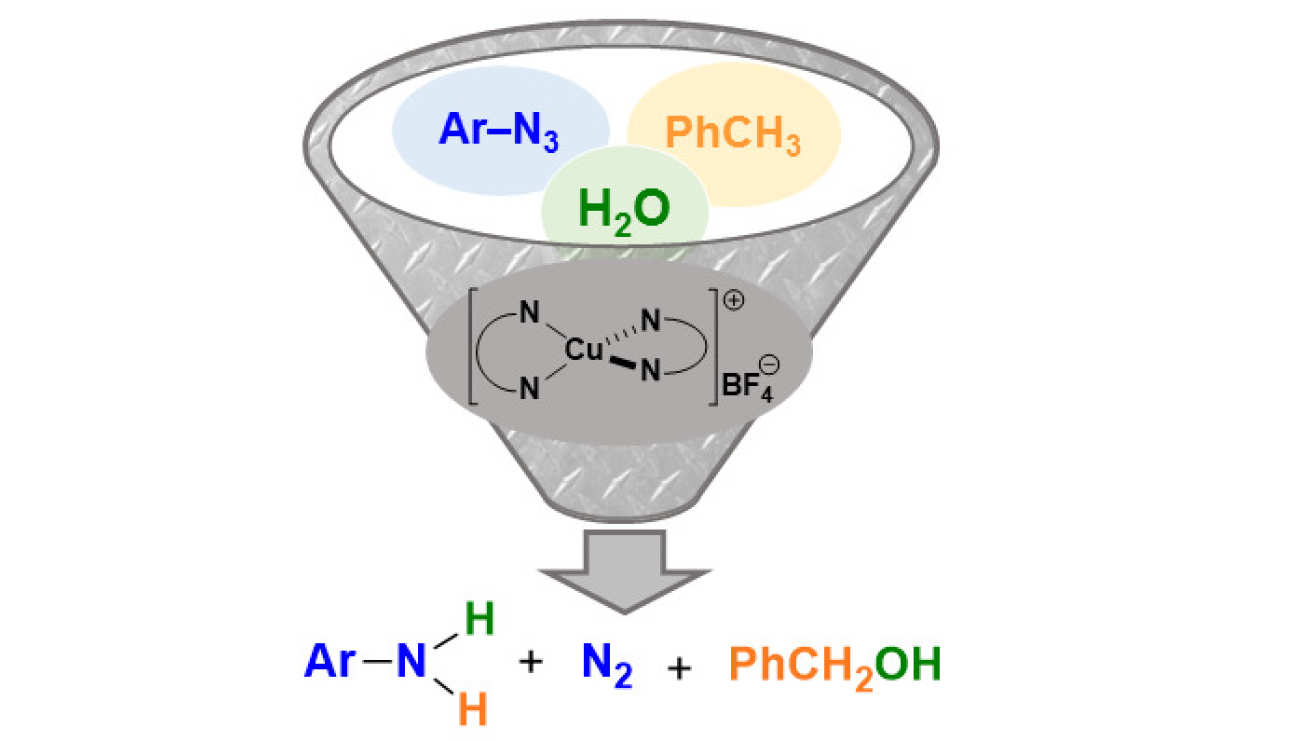
Zelenay, B.; Besora, M.; Monasterio, Z.; Ventura-Espinosa, D.; White, A. J.; Maseras, F.; Díez-González, S.
Catal. Sci Tech. 2018, 8, 5763–5773
The reduction of aryl azides in the absence of an obvious reducing agent is reported. Careful catalyst design led to the production of anilines in the presence of water and air. The reaction medium (toluene/water) is crucial for the success of the reaction, as DFT calculations support the formation of benzyl alcohol as the oxidation product. A singular catalytic cycle is presented for this transformation based on four key steps: nitrene formation through nitrogen extrusion, formal oxidative addition of water, C(sp3)–H activation of toluene and reductive elimination.
48. The acetate proton shuttle between mutually trans ligands
De Aguirre, A.; Díez-González, S.; Maseras, F.; Martín, M.; Sola, E. 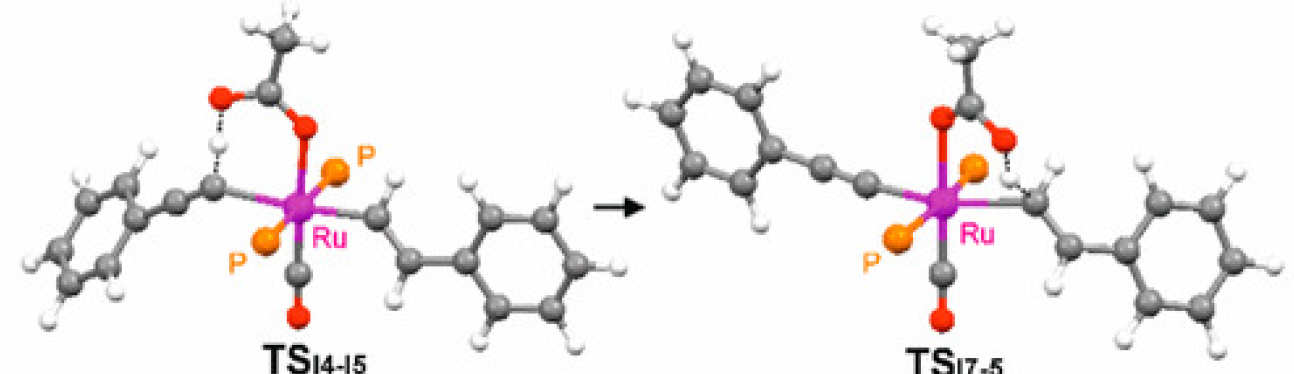
Organometallics, 2018, 37, 2645–2651
This work addresses a counterintuitive observation in the reactivity of the well-known ruthenium complexes [Ru(X)H(CO)(PiPr3)2], according to which the 5-coordinate chloro complex (X = Cl, 1) is less reactive toward phenylacetylene than its 6-coordinate acetate analogue (X = κO2-OC(O)Me, 3), since 3 undergoes a hydride-to-alkenyl-to-alkynyl transformation, whereas the reaction of 1 stops at the alkenyl derivative. The experimental kinetics of the key alkenyl-to-alkynyl step in the acetate complex are compared to the results of DFT calculations, which disclose the ability of the acetate not only to assist the alkyne C–H activation step via a CMD mechanism but also to subsequently deliver the proton to the alkenyl ligand. Possible consequences of this mechanistic resource connecting mutually trans ligands are briefly discussed on the basis of reported chemoselectivity changes induced by carboxylate ligands in 1-alkyne hydrosilylations catalyzed by this type of ruthenium complexes.
47. Thermal azide-alkene cycloaddition reactions: Straightforward multi-gram access to Δ2-1,2,3-triazolines in deep eutectic solvents
Sebest, F.; Casarrubios, L.; Rzepa, H. S.; White, A. J. P.; Díez-González, S.
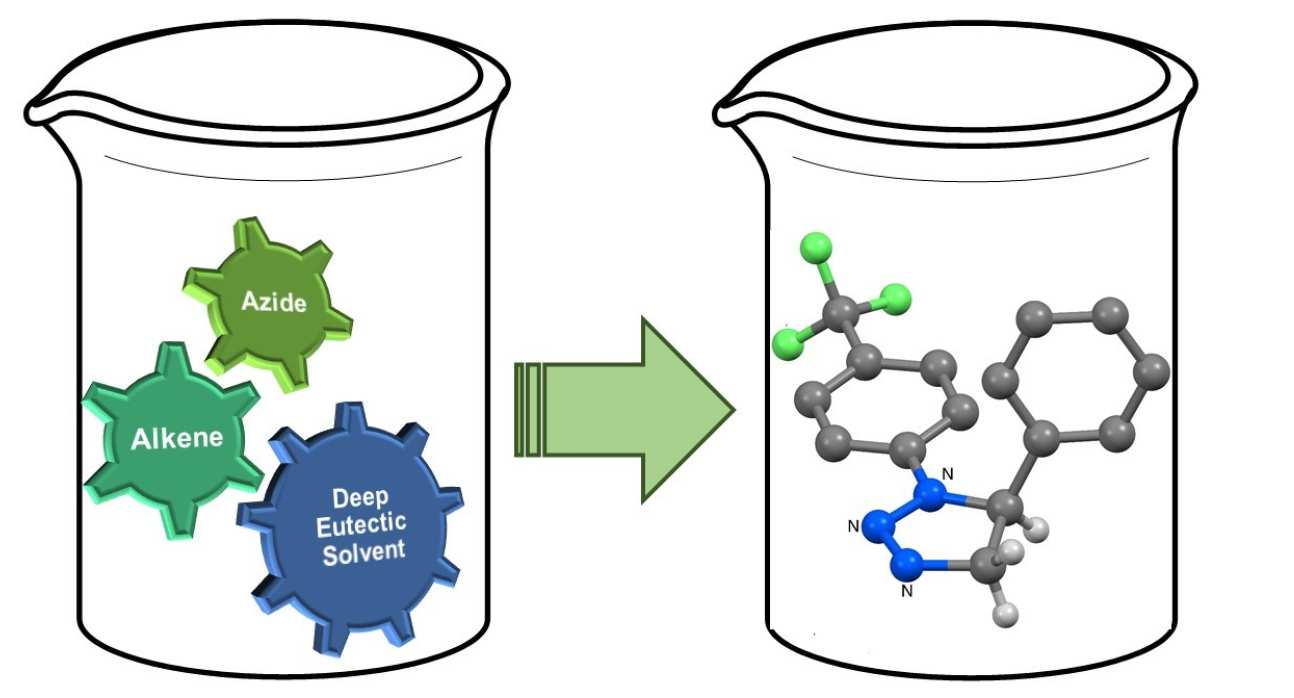
Green Chem. 2018, 20, 4023–4035
The multi-gram synthesis of a wide range of 1,2,3-triazolines via azide–alkene cycloaddition reactions in a Deep Eutectic Solvent (DES) is reported. The role of DES in this transformation as well as the origin of the full product distribution was studied with an experimental/computational-DFT approach.
46. Ring-expanded N-heterocyclic carbenes for copper-mediated azide-alkyne Click cycloaddition reactions
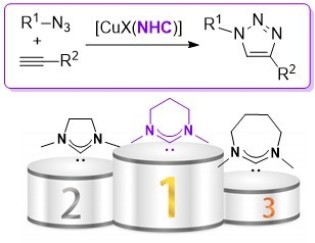
Sebest, F.; Dunsford, J. J.; Adams, M.; Pivot, J.; Newman, P. D.; Díez-González, S.
ChemCatChem 2018, 10, 2041-2045
In this article we studied different copper(I) complexes bearing ring‐expanded N‐heterocyclic carbene ligands in the azide–alkyne cycloaddition reaction. We showed that the six‐membered NHC ligands outperform well‐established five‐membered ones and [CuI(Mes‐6)] displayed a remarkable catalytic activity while respecting the strict criteria for click reactions.
45. Homo- and heteroleptic copper(I) complexes with diazabutadiene ligands: Synthesis, solution- and solid-state structural studies
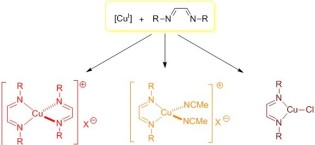
Zelenay, B.; Frutos-Pedreño, R.; Markalain-Barta, J.; Vega-Isa, E.; White, A. J. P.; Díez-González, S.
Eur. J. Inorg. Chem. 2016, 4649–4658
Herein we report the synthesis of several complexes with diazabutadiene complexes: [Cu(DABR)2]BF4), [Cu(DABR)(NCMe)2]BF4 and [CuCl(DABR)]. These complexes, which remain scarce in the literature are air-stable and their behaviour both in the solid state as well as in solution was studied by means of X‐ray crystallography, NMR and UV/Vis spectroscopy.
44. Copper(I)-acetylides: Access, structure and relevance in catalysis
Díez-González, S.
Advances in Organometallic Chemistry, 2016, Vol 66, 93-141
Pérez, P. J., Ed; Academic Press
43. Functionalised [(NHC)Pd(allyl)Cl] complexes: Synthesis, immobilisation and application in cross-coupling and dehalogenation reactions
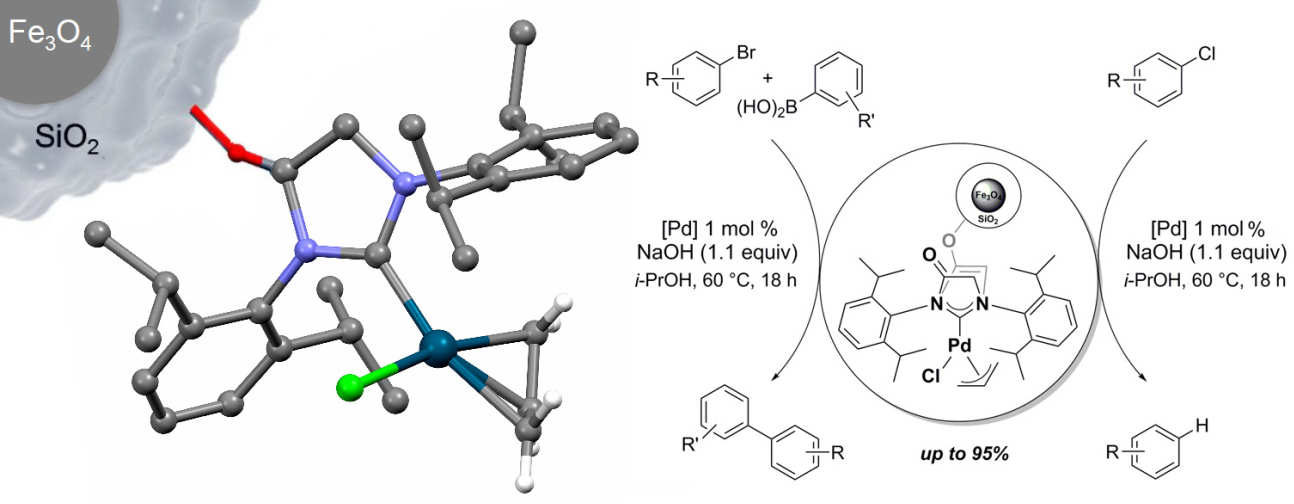
Collinson, J.-M.; Wilton-Ely, J. D. E. T.; Díez-González, S.
Catal. Commun. 2016, 87, 78–81
A novel NHC–palladium(II) complex and its immobilised version were prepared and fapplied in Suzuki-Miyaura cross coupling and chloroarene dehalogenation reactions. Also, an unexpected palladium-mediated transfer hydrogenation of a carbonyl compound was evidenced.


