Gene therapy for Parkinson's disease yields promising results in first patients
by Sam Wong
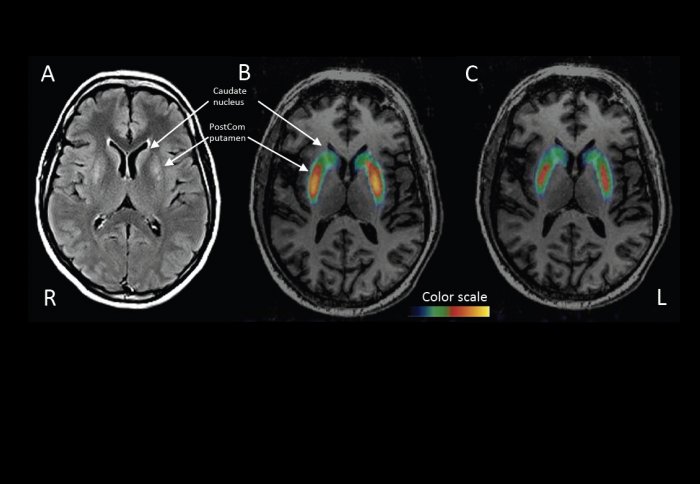
PET scans show higher dopamine levels in key brain areas six months after the treatment.
A new gene therapy for Parkinson's disease has achieved promising results in its first human tests, involving 15 patients.
Professor Nicholas Mazarakis, the Lucas Lee Chair of Molecular Biomedicine at Imperial College London, devised the approach while working at biopharmaceutical company Oxford BioMedica in 1997. Sixteen years later, the results of the first tests in humans have been published in The Lancet.
“It’s taken a long time to get to this point,” he says. “We’ve been fought all the way. People are hesitant to accept it as it’s so dramatic.”
The treatment, called ProSavin, uses a modified virus to deliver three genes into the striatum, a part of the brain that controls movement. The genes are intended to boost the production of dopamine, a chemical that becomes deficient in patients with Parkinson’s.
Current treatments can boost dopamine production temporarily, but the cells that produce dopamine continue to degenerate until the treatments are no longer effective.
I'm very pleased that it has appeared to work in the clinic. It has the potential to move to the next phase.
– Professor Nicholas Mazarakis
The new therapy aims to provide a long-term solution by stimulating dopamine to be produced in a different set of cells.
Other gene therapies have been tried in Parkinson’s disease, but all employ a different approach. Professor Mazarakis’s strategy is to deliver three genes that code for enzymes that produce dopamine. They are smuggled into the brain by a lentivirus, closely related to HIV, which incorporates its genetic material into the genome of the cells it infects, ensuring a long-lasting effect.
After first testing the treatment in rats, Oxford Biomedica worked with Professor Stéphane Palfi’s group at the University of Paris to carry out a study in macaques. While the study was ongoing in France, Professor Mazarakis received an unexpected phone call from his collaborator. “I can’t tell you what it is on the phone, but you’d better come over,” Professor Palfi said.
When he arrived in Paris, Professor Mazarakis saw monkeys that could barely move before the treatment climbing their cages.
“The surgeons couldn’t believe the effect,” he said. “Since then I knew that if the effect on movement in humans was as good as in primates, it would be something that can help people in late stage Parkinson’s disease, where they become very disabled.”
The team showed that the treatment corrected the movement deficits of the monkeys for up to three and a half years, without any visible adverse effects. Notably, it didn’t result in abnormal involuntary movements caused by treatments in use today.
Those results paved the way for the first tests in humans. Before beginning a double-blind, placebo-controlled trial straight away – the most rigorous type of trial – the team decided to test different doses on a small group of patients.
The trial participants, three in the UK and 12 in France, all in the advanced stages of the disease, underwent a single operation to inject the virus into the brain.
The treatment has been safe, with no serious adverse effects. The patients’ scores on movement tests have improved on average by 30 per cent, and they also report having a better quality of life. The first patients to have the surgery have now been followed up for four years, and the effect has been sustained. PET scans confirm that dopamine is being produced in the brain where it wasn’t before.
You have to remember that gene therapy is a field that's still maturing.
– Professor Nicholas Mazarakis
In such a small study, it’s difficult to compare the effects of different doses, but there are indications that the highest dose had the strongest effect. Patients on the highest dose all had to reduce their use of standard dopamine replacement therapy, as in combination with the gene therapy it caused side effects related to excessive dopamine.
The absence of a placebo group for comparison means the results have to be interpreted with caution, but the researchers are certainly optimistic.
“I’m very pleased that it has appeared to work in the clinic,” said Professor Mazarakis. “It has the potential to move to the next phase. It needs to be done in more people; we have to find the most effective dose, to further increase efficacy, and prove beyond doubt that this is not a placebo effect.”
The treatment is currently very expensive, but Professor Mazarakis expects that the cost will come down as gene therapies become more established and more companies produce viruses for gene delivery.
“You have to remember that gene therapy is a field that’s still maturing. The first trial was in 1990. Only now are we starting to see treatments getting close to the clinic.”
The team will continue to test the treatment on more patients, and plan to carry out a larger, placebo-controlled trial once they have optimised the dose and delivery method.
In the video below, from ParkinsonsMovement, Sheila Roy, a participant in the trial, describes how her condition improved after receiving the therapy.
Reference: S. Palfi et al. ‘Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial.’ The Lancet, 10 January 2014.
To find out about future clinical trials, please contact Oxford Biomedica or visit www.clinicaltrials.gov.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Sam Wong
School of Professional Development