Nanoscale 'baking tray' helps create crystals
by Kate Wighton
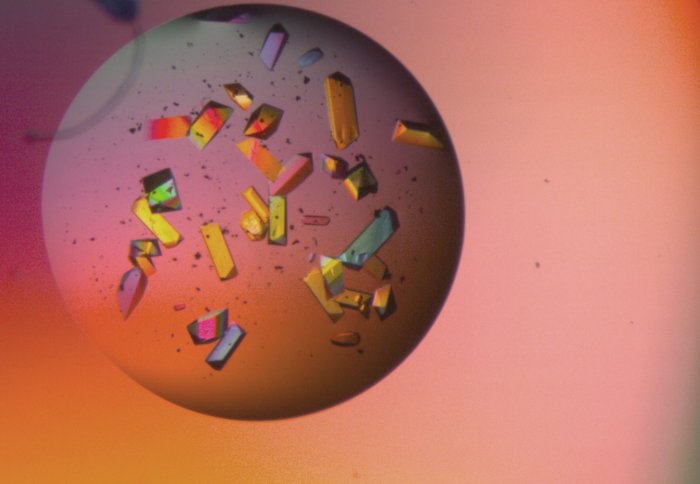
Colourful protein crystals viewed under a microscope. Each crystal is around 100 micrometres wide (similar width to human hair)
Scientists from Imperial College London have created a type of nanoscale 'baking tray' to help reveal the structure and shape of proteins.
The team, from the Departments of Surgery & Cancer, and Chemistry, hope this technology will enable new drugs and treatments to be developed.
Proteins are crucial to numerous reactions in the body - yet scientists are still in the dark about what most of them look like, explained Professor Naomi Chayen, lead author of the research.
All crystals start from a nucleation point. We see this is in many examples in life - this is why chains of bubbles in champagne or beer come from the same points on the glass
– Professor Milo Shaffer
Study author
"Results released by Structural Genomics consortia worldwide, show that we only know the 3D structure of around 20 per cent of the investigated proteins. Yet how can you target a protein that is involved in, say, cancer formation if you have no idea what it looks like? It’s like recognising a face in a crowd - you need a picture."
To obtain a picture of what a protein looks like, scientists need to persuade proteins - which are normally dissolved in liquid form, to grow into crystals. These crystals can then be examined with a beam of X-rays.
But triggering useful crystal formation is notoriously difficult – and this is the reason why we only know the structures of far fewer proteins than have been attempted, explained Professor Milo Shaffer: "All crystals start from a single point called a nucleation point. We see this is in many examples in life - for instance a snow flake starts at a single point, and it is why chains of bubbles in champagne or beer come from the same points on the glass - this is the nucleation point. After nucleation, the bubble or crystal can grow easily."
But reaching this nucleation point is often difficult, added Professor Shaffer: "Reaching this starting point requires a critical arrangement of the molecules - and many proteins can't overcome this hurdle, and so don't form crystals."
Once the molecules in the protein have arranged in a regular pattern, as a crystal, they can be analysed with X-rays. For years scientists been trying to find methods that will help proteins form crystals, but have struggled to find any technology or chemicals that is generally reliable and easy to apply.
In the latest technology, published in the journals Chemical Science and Nature Scientific Reports, the Imperial team harnessed the power of graphene. This so-called 'wonder material' is formed of a single layer of atoms, and has been proposed for a wide range of technologies from touch screens to satellites.
In the new study, the team attached long molecules, called polymers, to the graphene. These appear to define small pockets on the graphene surface, a bit like a baking tray. These grafted molecules help attract the proteins, and confine them in small regular clusters. This helps them to nucleate a crystal which can grow large enough to analyse using X-ray diffraction.
Now the technology has been shown to work, the team are investigating how they can adapt the system to work for different sizes and shapes of proteins, explained Professor Chayen.
"Now we know this technology works we are looking into making a type of universal crystallisation solution that can work for all types of experiments – and all types of proteins."
"Reductively PEGylated carbon nanomaterials and their use to nucleate 3D protein crystals: a comparison of dimensionality" by Leese at al is published in the journal Chemical Science.
"Exploring Carbon Nanomaterial Diversity for Nucleation of Protein Crystals" by Govada et al is published in the journal Nature Scientific Reports
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Kate Wighton
Communications Division