Enzyme’s ‘molecular scissors’ cut out fatal blood clot risk when injury strikes
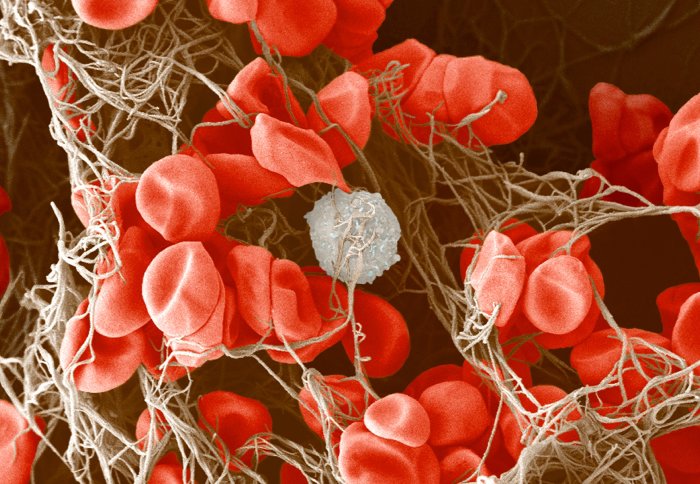
New research highlights how an essential enzyme works to prevent dangerous clots when blood vessels are damaged.
By stopping bleeding and allowing wounds to heal, our blood’s ability to clot is a vital part of how the body rebuilds and recovers following an injury. In rare cases, however, clots can continue to grow and prevent blood from flowing properly, leading to serious complications.
In a new study published in Nature Communications, researchers from Imperial College London, the University of Nottingham and KU Leuven have revealed fresh insights as to why this important biological process can sometimes go awry.
The findings show how a crucial enzyme in our blood, known as ADAMTS13, works like a pair of molecular scissors to carefully cut back the clotting effects of a key protein, von Willebrand factor (VWF).
While previous research demonstrated the important role of ADAMTS13 in regulating VWF, the findings pinpoint the specific molecular mechanisms underlying this crucial relationship by determining the crystal structure of the enzyme’s functional domains.
Cutting clot risk
"The mechanisms that we have uncovered beautifully exemplify how evolution has come up with elegant solutions to regulate complex biological systems" Professor James Crawley Department of Immunology and Inflammation, Imperial College London
When blood vessels are damaged, VWF binds to the site of injury and then unravels to form long protein strings that specifically capture specialised blood cells (platelets). This serves to stem the flow of blood and reduce bleeding. Patients who are deficient in VWF will continue to bleed, as in Von Willebrand disease. Conversely, if VWF is not regulated properly by ADAMTS13, it can result in thrombotic events such as heart attack, stroke or the life-threatening blood disorder thrombotic thrombocytopenic purpura (TTP). This is because when VWF is produced, it is hypersensitive to blood flow. Therefore, if VWF function is not adequately controlled this leads to excessive platelet clumping and clot formation.
Based on the study team’s exploration of the enzyme’s crystal structure, we now know that ADAMTS13’s unique butterfly-like shape allows it to bind specifically to VWF when it recognises the protein in our blood. Once bound, the enzyme acts as a pair of molecular scissors, trimming down VWF’s sticky protein strings and thereby preventing it from amassing blood cells excessively into a dangerous clot. After ADAMTS13 tailors the clotting effects of VWF, it reverts into a latent form which stops it from degrading other important proteins in our body. In this form, the enzyme also becomes resistant to inhibitors, allowing it to exist in our blood for longer.
Paving the way for new treatments
The study team is now keen to explore how this new knowledge of ADAMTS13’s highly-specialised structure can be used to develop treatments for strokes, heart attacks and rare blood disorders.
Senior author Professor James Crawley (Department of Immunology and Inflammation, Imperial College London) commented: “The results from our study provide a remarkable insight into how the body specifically controls blood clotting. The mechanisms that we have uncovered beautifully exemplify how evolution has come up with elegant solutions to regulate complex biological systems.”
“ADAMTS13 is one member of a family of enzymes, that all likely share a common mode of action. ADAMTS13 is the best-characterised member of this family. Therefore, our data not only provide important insights into human thrombotic diseases and potential novel therapeutics but also inform how the other ADAMTS family members may behave in many different and diverse biological systems.”
Co-Principal Investigator Professor Karen Vanhoorelbeke (KU Leuven) added: “Our study highlights the importance of combining expertise from different international research groups to realize scientific breakthroughs. Joining forces in determining crystal structures of proteins, enzyme kinetics and monoclonal antibody development allowed us to reveal how our body specifically controls this part of the blood clotting.”
Co-Principal Investigator Professor Jonas Emsley (Nottingham University) commented: “The pharmaceutical industry will benefit from this research through the potential to rationally modify and develop ADAMTS13 as a therapeutic agent. To do this, it is essential to understand its structure and function. To know which parts of the molecule are important (or redundant), and what the limiting steps in its production and function are central to facilitating this and design means to avoid recognition by the immune system,”
“The pharmaceutical industry is currently running trials of recombinant ADAMTS13 in the setting of thrombotic thrombocytopenic purpura (TTP). Work from this proposal will provide a molecular structure of ADAMTS13 in isolation and in complex with its substrate. Importantly, this will provide the opportunity to improve the ADAMTS13 molecule through targeted modification to reduce immunogenicity in inherited TTP, reduce inhibition and clearance in acquired TTP, and to reduce ADAMTS13 clearance and so prolong plasma half-life (improving bioavailability, reducing dosing/frequency of dosing).”
‘Crystal structure and substrate-induced activation of ADAMTS13’ is published in Nature Communications by Anastasis Petri, Hyo Jung Kim, Yaoxian Xu et al.
Image credit: Anne Weston, Francis Crick Institute
Image description: Colour-enhanced image of a blood clot. There are many red blood cells and a single white blood cell held together in a meshwork of fibrin (brown).
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Ms Genevieve Timmins
Academic Services