High resolution snapshots of NMT1 in action
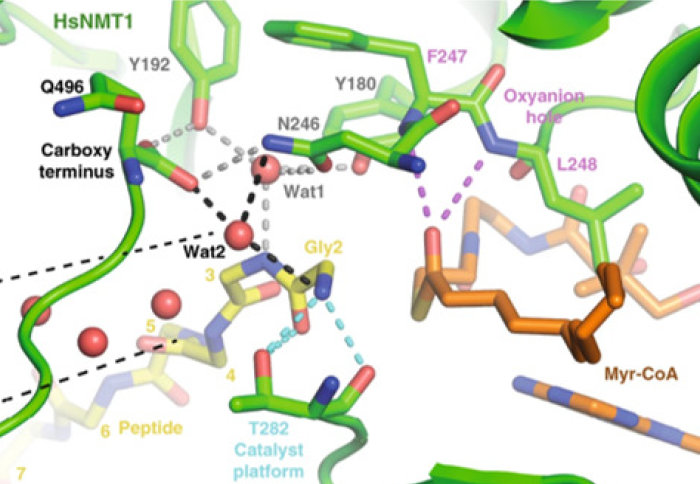
The Tate and Giglione groups have jointly published their collaborative study on NMT1, revealing the catalytic activity in detail
Research conducted in the Tate group by previous lab members Dr Inmaculada Perez-Dorado, Dr Markus Ritzefeld and Dr Jessica Shen, alongside researchers from the Giglione group of Université Paris-Saclay, has been published in Nature Communications.
NMT1 is a human protein that is known to transfer lipid chains of 14 carbons onto the ends of peptides, specifically onto glycine and sometimes lysine residues. This process is called myristoylation, or MYR. NMT1 is an essential component of the cell and is involved in many processes through MYR. NMT1 is also implicated in several diseases including cancer.
Dr Perez-Doraldo’s paper includes a series of structural snapshots at high resolution which reveal how the active region of NMT1 is poised during catalysis. These structures, generated by X-ray crystallography, clarify exactly how MYR occurs, and how the peptide substrate is modified. This information could be critical for the development of new chemical inhibitors of NMT1, with potential to impact numerous medical conditions.
This work was supported by Medicines for Malaria Venture and Cancer Research UK.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Edward Bartlett
Department of Chemistry