GENE DRIVE RESEARCH
GENE DRIVES AND MALARIA CONTROL
Historically, the most effective strategies to control malaria have targeted the mosquito vector rather than the malaria parasite. Insecticides and bednets that kill the mosquito or prevent infectious bites are the current frontline interventions for vector control and they have resulted in a dramatic reduction in malaria transmission over the last 20 years. Unfortunately these approaches are expensive, require a coordinated and sustained effort, and cannot be easily implemented across the whole of the malaria endemic world such as the worst affected regions of sub-Saharan Africa. New solutions are needed if malaria is to be eliminated entirely.
One potentially transformative strategy is the use of gene drives that can modify entire mosquito populations and reduce their capacity to transmit disease. Unlike other genetic modifications, gene drives spread by biasing their own inheritance. This makes them self-sustaining and able to reach areas that are currently inaccessible to vector control. Because they spread by mating they can be targeted to a single population or species, meaning that gene drives could be targeted to the few species that transmit malaria without affecting the 3500 other mosquito species that do not transmit the disease.
For more information, take a look at this review on gene drives for malaria control, written by myself and my colleague Dr Roberto Galizi.
How gene drives spread
Normal genes are inherited by half of the progeny - each progeny receiving one copy from their mother and one from their father. Whilst we could put new genes into the mosquito that might prevent them from transmitting malaria, these genes would not spread though a population. To do these, we have developed several technologies that are collectively known as "gene drive". A gene drive is able to spread through a population by biasing their own inheritance so that more than half of the progeny inherit it. The exact mechanism differs depending upon the strategy, and we have demonstrated a very effective method based upon endonucleases such as CRISPR. If the gene drive can bias transmission to more than 90% of the progeny, then it is expected that they could modify an entire population in under 2 years - even if just a few individuals that carry it are released.
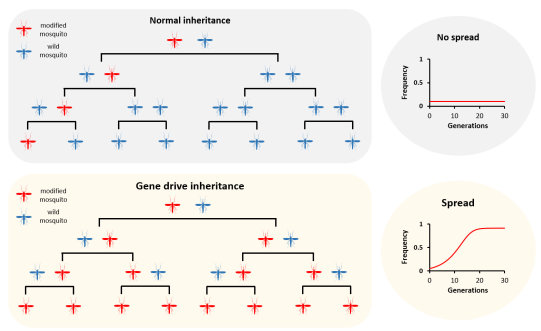
Without a gene drive, a modification gets inherited by only half of the offspring. Over time, the frequency of modified mosquitoes fails to increase. With a gene drive, a modification can duplicate itself so that all (or most) of the offspring inherit it. Over time, the gene drive will increase in frequency so eventually most or all of the mosquitoes will carry it.
tHE HOMING REACTION
In 2003, Professor Austin Burt was the first to suggest the use of endonucleases to make gene drives and by 2011, proof-of-principle had been demonstrated by the Crisanti lab. The strategy relies upon a reaction called “homing” that converts a modification from heterozygosity to homozygosity. To work, all that is needed is a highly specific endonuclease, a way to express that enzyme in the mosquito germline, and a suitable target site in the mosquito genome. Although we have shown that many endonucleases can function as gene drives, we have recently focussed upon CRISPR. My research on gene drives has been focussed on developing CRISPR-based gene drives for population control of the malaria mosquito.
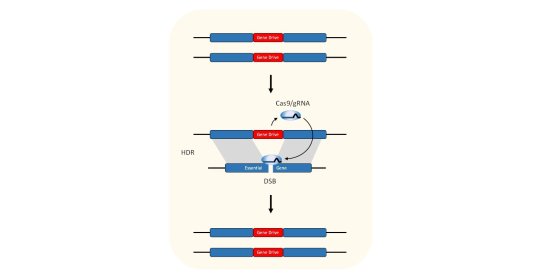
Gene drives contain Cas9 and gRNA that are programmed to recognise their insertion site homologous chromosome. In the germline of heterozygotes, Cas9 and gRNA are expressed, where they will cleave the homologous chromosome. This broken chromosome is usually repaired using the unbroken chromosome as a template for repair, in a process called homology directed repair (HDR). This causes the gene drive to be copied inside the broken chromosome so that it is now homozygous. This reaction, called homing, means that the gene drive no longer transmitted to half of the offspring (as one would expect of a gene that is heterozygous), but instead transmits to almost all of the progeny.
Population suppression with gene drives
To suppress or eliminate a mosquito population, Burt postulated that the most effective gene drive should be targeted to disrupt a gene needed by female mosquitoes to reproduce. This “target gene” needed to fulfil several criteria to ensure that the gene drive could spread through the mosquito population before making them sterile (at first glance this appears to be paradox, how does a gene drive spread into the progeny if the females are sterile!? – see the figure below to help this make sense). Much work had been done by the groups of Crisanti, Burt and Russell to find these genes and when I joined the team in 2012, I was tasked with finding and validating these genes that might be needed for female development or reproduction in the mosquito.
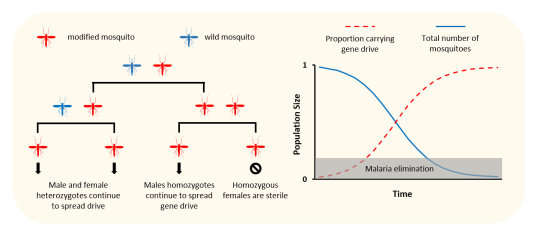
A gene drive can be designed to suppress a mosquito population if it makes female mosquitoes infertile. To work, it must only make female mosquitoes infertile if both of its parents carry the drive. As it spreads, more and more mosquitoes will be made infertile and the population will reduce or crash. Not all mosquitoes need to be killed to stop malaria - if the number of mosquitoes is brought below a critical threshold, malaria transmission will fall too low and the disease will be eliminated.
STATE of the art
Shortly after I joined the team, CRISPR technology burst onto the scene and we saw an incredible opportunity to quickly verify potential target genes using CRISPR to disrupt them with a fluorescent marker protein called GFP. We knew that if CRISPR worked well, it could be re-programmed to work as a gene drive and designed a system that could quickly convert the GFP into a gene drive. In 2015, we published my primary PhD research demonstrating the first gene drive system designed to suppress populations of the malaria mosquito, including the first use of CRISPR in the African malaria mosquito. In caged release experiments we observed these gene drives spreading, but soon afterwards the mosquitoes were able to force the gene drive out of the population as a result of evolved resistant mutations. These mutations were predicted in Burt’s original theory and I set about understanding the nature of their creation and selection, publishing this first study of evolved resistance to gene drive in 2017. To overcome this problem, we decided to focus upon targeting sites in the genome that might be unable to tolerate the types of mutations that had previously been shown to confer resistance. Working with a PhD student in lab, Kyros Kyrou, we identified an extremely conserved site in the a region of the doublesex gene that is required for female development and built a gene drive designed to target this. By building in additional improvements to the drive design, it was able to spread very quickly through caged populations, without selecting for resistance, and ultimately eliminating the entire mosquito populations. The lab is now testing these strains on a larger scale, to see if they are likely to work in a wild population of mosquitoes in Africa. We are also developing the technology further in anticipation that, given enough time, resistance could still arise in a larger population.

