Research interests
My research activity focuses on understanding the physicochemical and electrochemical processes that control the operation of energy conversion and storage systems. This involves developing multiphysics models at different scales which can be combined in hierarchical approaches to identify the interplay of various phenomena involved and quantifying the relevant processes, helping to distinguish key phenomena. This is done through the development of mathematical models that describe the phenomena involved and provide a good estimate of the operational performance of these systems. Experimental observations of the main operational and output variables are obtained to parametrise, calibrate and validate these models.
Currently, my work focuses on cell level models for redox flow batteries such as all-vanadium, hydrogen-vanadium, hydrogen-manganese, and non-aqueous RBFs, and hierarchical models for solid oxide cells, such as electrolyte and anode supported cells.
Solid Oxide Cells

Solid oxide cells convert electrical energy to chemical energy, and vice versa, operate at high temperatures (600–1000°C) and have exceptional conversion/energy efficiencies.
When SOCs operate in fuel cell mode (SOFCs), they produce electrical energy by directly oxidizing a fuel. These high-temperature fuel cells have the ability to run on fuels other than hydrogen. This energy can be considered environmentally friendly when using green hydrogen (or other fuel) as reactants.
When SOCs operate in electrolysis mode (SOECs) they produce hydrogen and oxygen from water when electricity is supplied, perhaps coming from renewable sources such as solar and wind. Co-electrolysis of H2O and CO2 is another possible application.
They can also operate in both modes reversibly and these devices are called reversible SOCs (rSOCs). SOCs have three main ceramic components. Two composite electrodes each contain electric and ionic conducting phases and a solid oxide electrolyte which is an ionic conducting phase.
Featured research
High efficiency reversible solid oxide cells for the integration of offshore renewable energy using hydrogen (EP/W003597/1)
Named researcher leading the reversible Solid Oxide Cell (SOC) stack modelling (Mar 2022 - Present)
to be continued
Hybrid ML/physics-based hierarchical modelling of rSOC
to be continued
Presentations
H2FC Research Conference, St Andrews, UK, June 8-9, 2022. Poster presenter: A physics-based data-driven model for electrolyte supported SOCs.
Redox Flow Batteries

Redox flow batteries are strong candidates for grid-scale energy storage applications due to their potential to decouple power and energy capacity. This means that they allow for flexible system design to meet desired storage applications more easily than other battery technologies. Redox flow batteries store or deliver energy by means of electrochemical reactions, i.e., reduction-oxidation (redox) reactions, that occur at electrodes and change the oxidation state of electro-active species. This particular kind of battery uses two electrolyte solutions, which are stored in external tanks and contained the electro-active species that participate in the electrochemical reactions when these solutions are pumped into the electrochemical cell. Hybrid-type RFBs such as hydrogen-halogen are of interest due to their fast reversible kinetics and the facile separation of crossover species if present.
Featured Research
System-level modelling of hybrid redox flow batteries
Hybrid hydrogen-X RFBs, also known as Regenerative fuel cells (RFCs), could offer pathways to reduce the costs of redox flow batteries. These systems require further increases in energy and power efficiencies to become competitive with other electrical energy storage technologies (e.g., Li-ion batteries). The aim of this project is to develop a model, specifically at the stack/system level, that could be used to investigate the performance and cost of different RFCs, such as H2/V RFC, H2/BQDS RFC, and H2/Mn RFC. The model will be used for design optimization and control/monitoring-oriented applications.
to be continued.
Membrane transport in hybrid redox flow batteries
to be continued
Physics-based optimisation of Organic RFB using Gaussian Processes and Bayesian optimisation
To be continued.
Effect of flow fields on a regenerative hydrogen-manganese fuel cell
To be continued.
Understanding electrode transport in an organic redox flow battery
This international collaboration between the Brushett group at MIT and the Brandon group at Imperial College aimed to understand and unpick the relationship between electrode microstructure and redox flow battery performance. A broad approach has been taken by using a wide range of analysis techniques such as X-ray computed tomography, electrochemical impedance spectroscopy and two types of model simulation, to separate out and decipher the dominant transport processes in a redox flow battery system. The outcomes aim to be as far-reaching and applicable as possible to liquid flow-through-type flow cells. Microstructural properties are shown to have unexpected and widely varying influences on performance and certain properties are shown to have a significant impact on cell performance.
My main contributions were on project supervision for Imperial College and the continuum modelling work to simulate the symmetric cell polarization response, along with the characteristic number study from the non-dimensionalisation analysis.[1]
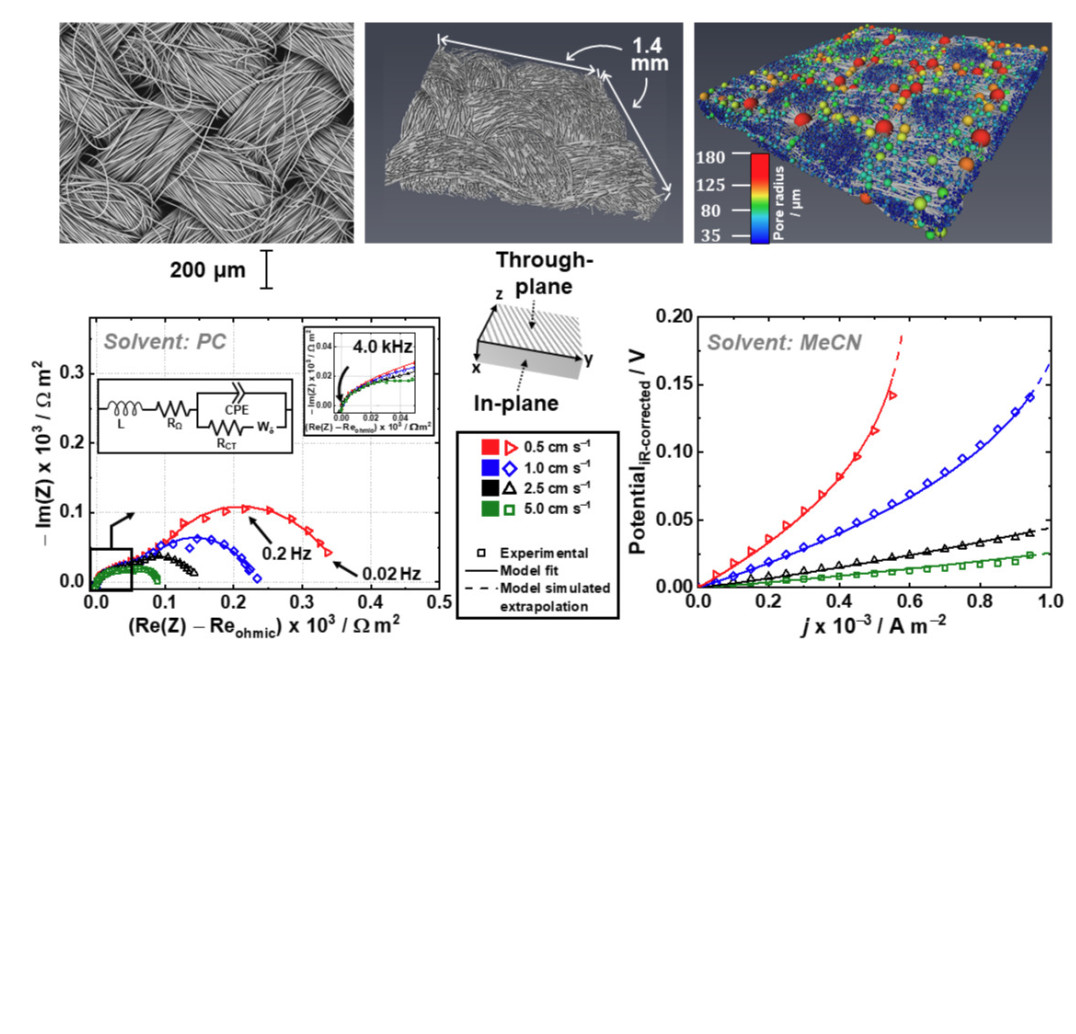
Top row: SEM, XCT and pore network extracted from a carbon (cloth) electrode tested in this study. Bottom row: Impedance and polarisation analysis at various flow velocities in propylene carbonate and acetonitrile, respectively.
Reduced-order models for a Regenerative hydrogen-vanadium fuel cell
A regenerative hydrogen-vanadium fuel cell (RHVFC) benefits from the advantages of the mixed liquid-gas RFBs and reduces the overall system cost by utilising only half of the vanadium electrolyte required for a VRFB. However, they have faced challenges related to cost, scale-up and optimisation. A better performance could be obtained if improved component materials and operating conditions were used. Modelling and simulation are indispensable tools, saving time and reducing costs. This work, developed as part of my PhD project, introduced a 0D model for a unit cell of an RHVFC.[2,3] The model was based on the physicochemical phenomena explained by mass conservation, transport mechanisms and electrochemical processes, but maintained simplicity to allow its use in monitoring and designing. A thorough characterisation of the cell performance and a study of the effect of properties and operating conditions were developed by using the reduced order model.
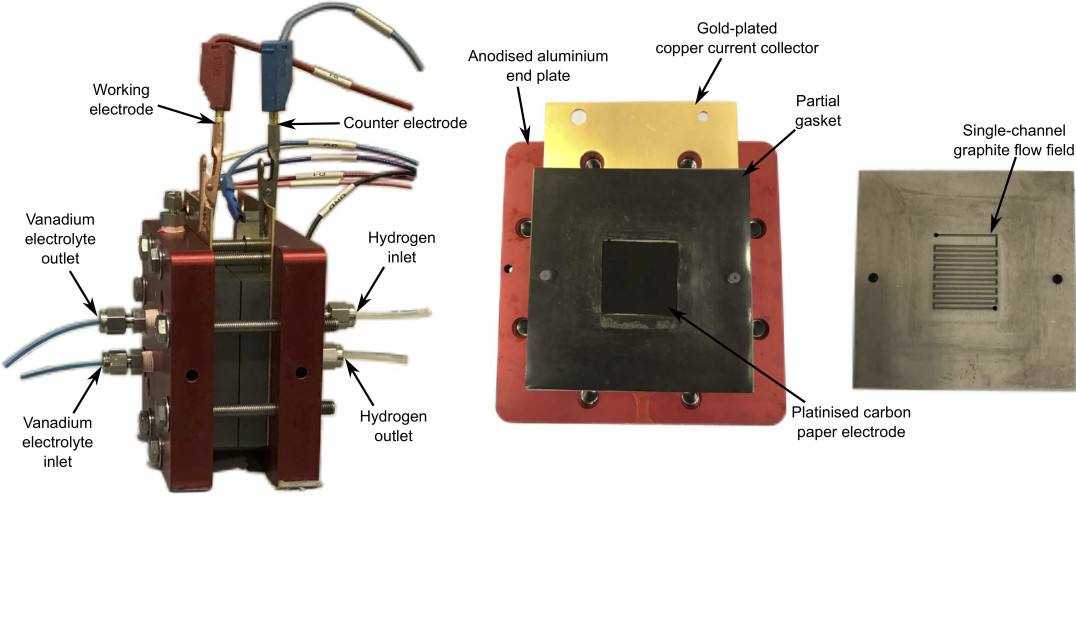
RHVFC system and its components
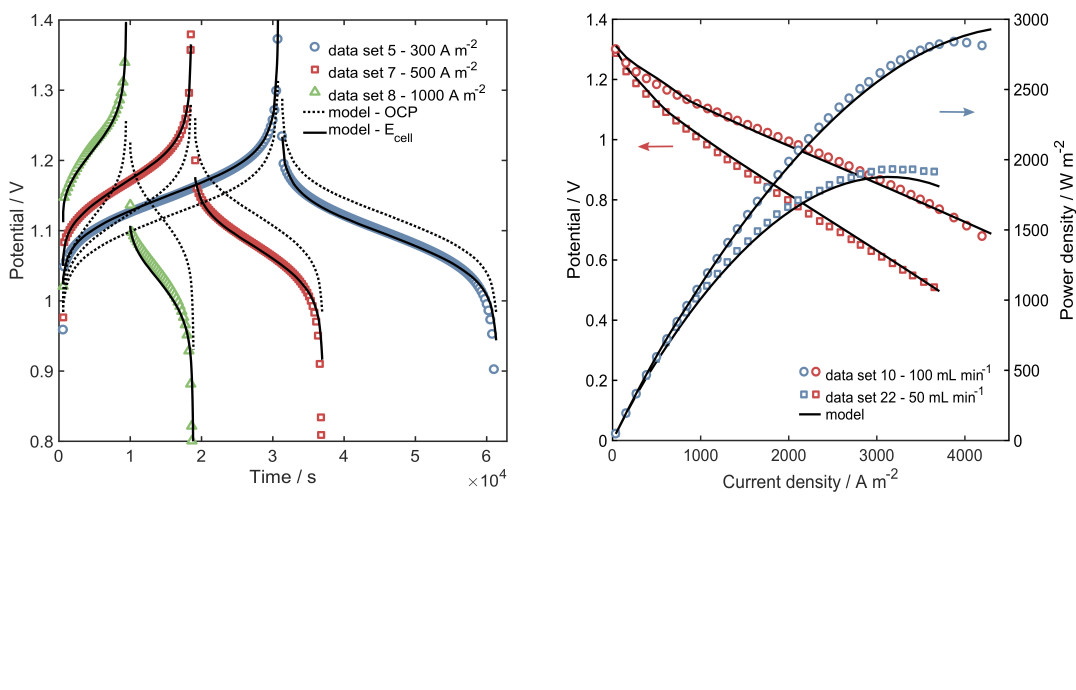
Single-cycle charge-discharge potential (left), and polarisation and power curves (right)
Published work
- Simon BA, Gayon-Lombardo A, Pino-Muñoz CA, Wood CE, Tenny KM, Greco KV, Cooper SJ, Forner-Cuenca A, Brushett FR, Kucernak AR, Brandon NP, 2022, Combining electrochemical and imaging analyses to understand the effect of electrode microstructure and electrolyte properties on redox flow batteries, Applied Energy, Vol:306, ISSN:0306-2619, Pages:1-22.
- Pino-Muñoz C. A., Hewa-Dewage H., Yufit V., , 2017, Unit cell model of a regenerative hydrogen-vanadium fuel cell, Journal of the Electrochemical Society, Vol:164, Pages:F1717-F1732, ISSN:0013-4651
- Pino-Muñoz C. A., Chakrabarti B. K., Yufit V., , 2019, Characterization of a regenerative hydrogen-vanadium fuel cell using an experimentally validated unit cell model, Journal of the Electrochemical Society, Vol:166, Pages:A3511-A3524, ISSN:0013-4651.
Presentations
Electrochemical Science and Engineering 2021 Showcase. Oral presenter: Physics-based modelling for hybrid redox flow batteries
SUPERGEN Energy Storage Consortium Meeting, Oxford, UK, 26 November 2019. Oral presenter: Modelling of a Regenerative Hydrogen-Vanadium Fuel Cell.
XII International Conference on Computational Heat, Mass and Momentum Transfer, Rome, Italy, 3-6 September 2019. Oral presenter: Modelling of membrane transport in a regenerative hydrogen-vanadium fuel cell.
Modval 2019, Braunschweig, Germany, 12-13 March 2019. Oral presenter: Characterisation of a regenerative hydrogen-vanadium fuel cell using an experimentally validated unit cell model.
232nd ECS meeting, National Harbor, M. 1-5 Oct 2017. Oral presenter: Unit cell model of a Regenerative Hydrogen-Vanadium Fuel Cell.
SCI Electrochemistry Postgraduate conference, University of Southampton, UK, 19 May 2017. Oral presenter: Unit cell model of a Regenerative Hydrogen-Vanadium Fuel Cell.
UKES conference, University of Birmingham, UK, 30th Nov - 2nd Dec 2017. Poster presenter: Unit cell model of a Regenerative Hydrogen-Vanadium Fuel Cell.
IFBF, Karlsruhe, Germany, 7th-9th June 2016. Poster presenter: Unit cell model of a Regenerative Hydrogen-Vanadium Fuel Cell.
Photoelectrochemical water splitting

Project
“Highly stable organic and perovskite photoelectrodes for water splitting using electrospun conductive nanofiber network”, Aug 2022 - Jul 2023, Co-I.
Funding
Dame Julia Higgins Postdoc Collaborative Research Fund, Imperial College.
Guest Lectures
Energy Futures Lab. MSc Sustainable Energy Futures, course: Fuel Cell and Electrolyser Modelling and Systems I & II, Imperial College London, London, UK, 2021
Energy Futures Lab, MS Sustainable Energy Futures, course: Thermodynamics of Fuel Cells and Electrolysers, Imperial College London, London, UK, 2021
Energy Futures Lab. MSc Sustainable Energy Futures, course: Introduction to Fuel Cell Modelling I & II, Imperial College London, London, UK, 2020
Energy Futures Lab, MS Sustainable Energy Futures, course: Fuel Cell Thermodynamics, Imperial College London, London, UK, 2020
Energy Futures Lab. MSc Sustainable Energy Futures, course: Fuel cell systems and modelling I & II, Imperial College London, London, UK, 2019
Research Student Supervision
Machida,T, Hybrid wind based Power-to-Hydrogen (P2H2) production optimisation (Co-supervised with Anthony Wang, ETFuels), Feb 2023 - Present.
Wicaksono,G, Hybrid geothermal based Power-to-Ammonia (P2A) production optimisation (Co-supervised with Anthony Wang, ETFuels), Feb 2023 - Present.
Ye,M, System-level modelling of hybrid redox flow batteries (Co-supervised with Professor Nigel Brandon and Professor Anthony Kucernak), May 2021 - Present.


