Overview
My current research centres on finding answers to the question ‘How can we continue to use fossil fuels for most of this century (as I believe we must) without causing catastrophic climate change?’. The work aims to provide solutions for managing the transition from oil, gas and coal to more sustainable, renewable energy sources and vectors. A common thread running through all my research interests is making the links between interactions at the molecular/colloidal level and the bulk properties of materials and then to exploit this to give accurate data for fluid properties which provide solutions for industrial problems – Fluids Engineering. The research forms part of the Physical Properties and Analytics Laboratory, with links to several of the Department’s research themes: Energy/Sustainability, Molecular/Materials and Multiscale Driven Chemical Engineering.
Please note that I am no longer taking new PhD students or Postdoctoral Researchers.
The research is built around three main themes:
(a) Carbon Capture and Storage
My research within the Qatar Carbonates and Carbon Storage Research Centre, QCCSRC, of which I was the founding Director, and more recently the Shell-Imperial Digital Rocks Programme, focuses on making accurate experimental measurements for CO2-brine-hydrocarbon fluids under HPHT reservoir conditions of the thermophysical properties which determine how CO2, and its mixtures with other co-injected gases, behaves when it is injected into an underground storage site, mixes with the in situ reservoir fluids then flows and reacts over long periods of time within the porous/fractured carbonate rock minerals that constitute the reservoir. The philosophy is to make measurements at selected temperatures, pressures and fluid-rock compositions relevant to carbon storage processes and then to use these data to calibrate, validate and use predictive models to enable the required properties of fluid mixtures of arbitrary composition to be predicted as a function of temperature and pressure as they move through a reservoir. The research has been done in close collaboration with Professor J P Martin Trusler. The most powerful predictive models are based on simplified molecular models incorporated within the framework of statistical mechanics (for equations of state and phase behaviour) and advanced kinetic theory (for transport properties). These models have been developed by Professor George Jackson, Professor Amparo Galindo and Professor Velisa Vesovic and we have worked with them on applying them to both model fluid mixtures and complex, real reservoir crude oils and brines.
The main properties of interest are:
- Vapour-liquid phase behaviour
- Interfacial tension and fluid-mineral contact angles
- Transport properties: viscosity and diffusion
- Mineral-CO2 reaction kinetics
The equipment is custom-designed and built within the Department and produces data of high precision. The skills of the Departmental Mechanical Workshop are critical to achieving the research goals. More details of the Thermophysics Lab are given here. We have worked with other groups within Chemical Engineering and Earth Science and Engineering (particularly Professor Martin Blunt) on how the data and predictive models for these properties may be incorporated into pore-scale models and reservoir simulators for CO2 storage design.
Relevant research projects (jointly with Professor Martin Trusler) include:
- Phase behaviour, solubilities and densities of impure CO2 mixed with model and reservoir brines at high temperatures and pressures (Rayane Hoballah)
- Interfacial tension and contact angle measurements for CO2 and impure CO2 mixtures with water, brines and hydrocarbons at high temperatures and pressures (Florence Chow)
- Diffusion of CO2 in water and hydrocarbons at high temperatures and pressures (Shane Cadogan)
- The pH of CO2 dissolved in water and brines and the reaction of CO2 with carbonate minerals at elevated temperatures and pressures (Cheng Peng)
- The viscosity of CO2 and its mixtures with impurities in model and reservoir brines at high temperatures and pressures (Claudio Calabrese)
- The reaction of CO2 with carbonate minerals and rocks in the presence of gaseous and mineral surface impurities (Benaiah Anabaraonye)
Other completed projects carried out in QCCSRC include:
- Interfacial Tension of Aqueous and Hydrocarbon Systems in the Presence of Carbon Dioxide at Elevated Pressures and Temperatures (Apostolos Georgiadis, 2011)
- Measurement and Prediction of the Viscosity of Hydrocarbon Mixtures and Crude Oils (Faheem Ijaz, 2011)
- Interfacial Properties of Reservoir Fluids and Rocks (Xuesong Li, 2012)
- Phase Behaviour and Physical Properties of Reservoir Fluids Under Addition of Carbon Dioxide (Saif Al Ghafri, 2013)
- Viscosity and Density of Aqueous Fluids with Dissolved CO2(g) (Mark McBride-Wright, 2013)
In the carbon capture area, I have collaborated with Professor Paul Fennell on developing more efficient and cost-effective carbon capture processes. Recent projects include:
- Use of calcium looping systems in pyrolysis and combustion of biomass (Joseph Yao 2017)
- Development of Advanced Amine Systems with Accurate Vapour-liquid Equilibrium Measurement (Danlu Tong 2012)
(b) Understanding and exploiting the use of gas hydrates
I am interested in better understanding the thermodynamics and kinetics of gas hydrate formation, through both experimental and modelling studies at the elevated pressures and low temperatures at which naturally occurring hydrates exist or synthetic gas hydrates can be formed, either during gas recovery and transportation operations or as a means to separate or store gases. Techniques used to study gas hydrate phase behaviour, nucleation and growth and gas exchange include micro-DSC (Differential Scanning Calorimetry), a customised stirred autoclave with optical window and Raman spectroscopy and 3D imaging.
The formation and decomposition of methane hydrates have been studied in detail (by Dr Hao Bian, PhD 2017) using different aqueous precursors: bulk water, micronised ice and 'Dry Water', a Pickering emulsion of 100 micron-sized water droplets stabilised using hydrophobic nanoparticles which contain about 95 wt% water but flows like a solid powder. These studies have demonstrated that modifying water precursor structure can dramatically increase gas hydrate formation rates and yields. The effect of pore confinement and surface wettability on hydrate phase behaviour has also been studied, mimicking in model systems the type of conditions that hydrates form in subsurface mineral sediments.
This improved understanding of the dynamics of hydrate formation, dissociation and guest gas exchange is being used to explore and design processes which exploit gas hydrates for a range of applications, including:
- Injection of CO2 into natural CH4 hydrates to store CO2 in the subsurface (as part of CCS) and tap into the vast reserves of methane available in gas hydrates as a source of the cleanest fossil fuel needed (again with CCS) as a source of blue hydrogen and syngas during the energy transition towards net-zero carbon emissions;
- Separation of close boiling point gas mixtures, such as propane-propene, with a much lower energy requirement and carbon footprint than traditional cryogenic distillation methods (PhD student Lu Ai, co-supervised with Professor Klaus Hellgardt);
- Storage of large volumes of gases, particularly hydrogen, in solid form as an alternative to liquefaction or high pressure tanks, for transportation and applications such as fuel cells.
(c) Combining CO2 storage with enhanced gas recovery and low-carbon footprint subsurface processing for hydrogen and syngas production
My research is seeking ways to recover and utilise hydrocarbons, including non-conventional resources, in ways that provide gas required for engineering an affordable and equitable energy transition, which minimise the energy input required and the CO2 emissions from the processes. The areas covered include:
- Subsurface processing of residual oil in depleted conventional reservoirs as well as heavy oil and oil shales, combined with in situ carbon capture and storage, to produce only hydrogen and syngas as surface products;
- The production of non-conventional gas (gas hydrates, shale gas) using CO2 injection to enhance methane production before being sequestered in the producing formation.
A long-term aim of this research is novel integrated processes for clean, sustainable production of non-conventional fossil fuels, especially gas hydrates and heavier crudes. I am exploring with Professor Klaus Hellgardt, Professor Sandro Macchietto and others the possibilities of exploiting the inherent thermal and pressure energy of in situ reservoir fluids and of using the long high temperature, high pressure underground well network for sub-surface separation and chemical conversion processes, alongside downhole removal and sequestration of low value and polluting materials such as CO2 and H2S. The overall aim is to transform the routes by which we extract and process fossil fuels so that power, clean fuels (e.g. hydrogen) and chemical feedstocks (e.g.syngas), in appropriate combination, are the dominant process outputs, with produced CO2 captured and reinjected for storage in the reservoir without being released to the surface.
Recent projects include:
- Production, Stabilisation and Carbon Capture for Gas Hydrates (Dr Hao Bian) (with Professor Klaus Hellgardt and Professor Jerry Heng)
- Properties and Production of Natural Gas Hydrates (Dr Udennaka Paul Igboanusi, PhD 2009)
CO2 enhanced recovery of methane from shales has been studied experimentally combined with adsorption and process modelling by Dr Humera Ansari (PhD 2021, co-supervised with Dr Ronny Pini and Professor Martin Trusler). The principal use of the produced methane will be for cleaner power production and provision of 'blue' hydrogen via steam methane reforming, both processes being decarbonised using CCS.
(d) Renewable production of hydrogen using green algae and cyanobacteria
The most likely long-term source of global energy will be solar, where capturing but a small fraction of the energy reaching the earth’s surface will meet all our energy needs on a continuing basis. As well as converting solar radiation to electrons for electricity supply, it may also be directly converted to fuels and chemicals. My research is this area, carried out in collaboration with Professor Klaus Hellgardt, has been investigating processes for the direct production of hydrogen (as a zero-carbon fuel or energy vector) from water using sunlight and the enzymatic conversion pathways embedded in natural micro-organisms such as green algae and cyanobacteria. The work investigates both the underlying mechanisms and the design of photo-bioreactors at different scales to explore the possibility of large-scale commercial hydrogen production processes based on this approach.
The research has investigated the effect of parameters such as light intensity, illumination patterns, the type and control of nutrients (such as sulphate or nitrate deprivation to achieve anaerobic conditions) as a means of understanding better the underlying mechanisms and enabling the design of photo-bioreactors at different scales to explore the possibility of large-scale commercial hydrogen production processes based on this approach. A major challenge has been to control nutrient addition in such a way that hydrogen is produced continuously rather than peaking and decaying as the micro-organisms die or fail to maintain their anaerobic condition. A novel two-stage chemostat approach was developed by which we can achieve continuous co-production of H2 and biomass in the laboratory. This may provide a route to producing continuous hydrogen in significant quantities at the pilot and larger scales. The research has been funded by a £4m EPSRC Solar Hydrogen grant (2007-2012) of which we were both co-investigators (see solar routes to hydrogen).
The initial project investigating green algae was:
- A study of the growth and hydrogen production of Chlamydomonas reinhardtii (Dr Bojan Tamburic, PhD 2012)
A subsequent project investigated cyanobacteria:
- Biophotolytic H2 and 1,3-propanediol production by a unicellular N2-fixing cyanobacterium Cyanothece sp. ATCC 51142 (Dr Pongsathorn Dechatiwongse, PhD 2015)
(e) Energy Engineering Thermodynamics
I have carried out detailed thermodynamic analyses of many of the processes associated with the energy transition and decarbonisation of power, heating, transport and industrial processes, including carbon capture and storage (CCS), hydrogen production, renewable energy production and energy storage processes. These are described in the book:
'Commonly Asked Questions in Thermodynamics - Second Edition' by Marc J. Assael, Geoffrey C. Maitland, Thomas Maskow, Urs von Stockar, William A. Wakeham, and Stefan Will, published by CRC Press (Taylor and Francis Group), August 2022.
This is a useful text for undergraduates, postgraduates, energy practitioners and even for people with a non-engineering background interested in understanding the relative merits of different energy vectors and the challenges of decarbonising the energy system. Here is a table of heat capacities used in Chapter 6 to calculate changes of enthalpy with temperature if you wish to reproduce those calculations.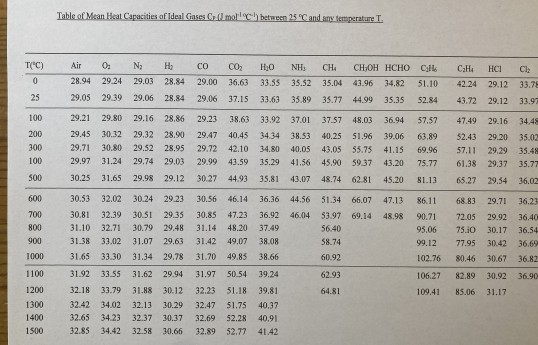
Research Student Supervision
Ansari,H, CO2 enhanced shale gas recovery
Jones,C, Quantification and monitoring of fluid phase behaviour and trapping in geological carbon sequestration sites

