Overview
Current research topics in the Nixon group:
1) ENHANCING PHOTOSYNTHESIS
In the next three decades the global demand for food is expected to double. However, current trends in crop yield are unlikely to be sufficient to meet this demand. Plant photosynthesis, which drives plant growth, is a relatively inefficient process so one approach is to engineer crops with enhanced photosynthetic performance. Our current interest is focused on improving the efficiency of the photosynthetic electron transport chain that converts solar energy into chemical energy. One target is to introduce novel chlorophyll pigments that absorb in the far-red region of the spectrum. A second is to enhance the production of ATP.
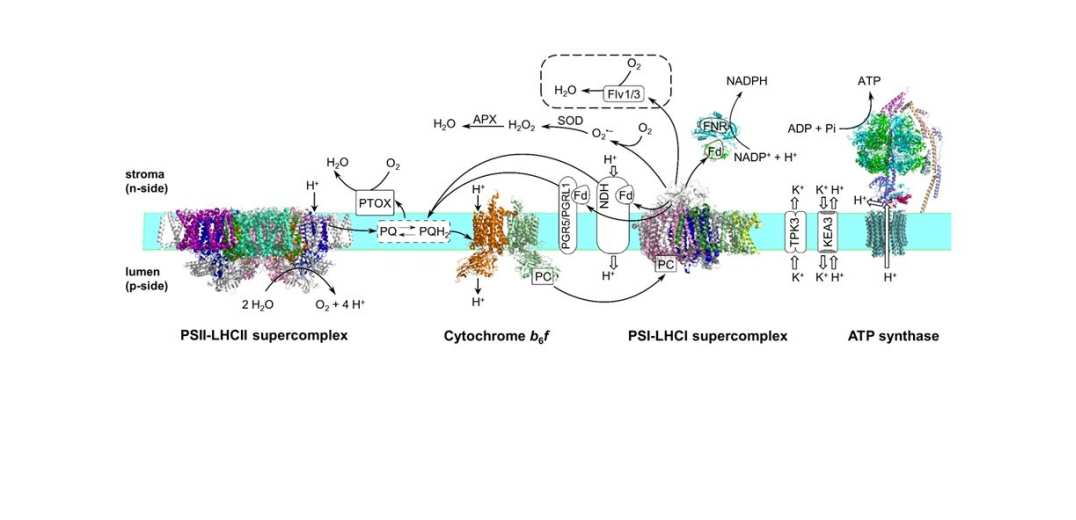
Figure 1: Photosynthetic electron transport
2) Assembly and repair of Photosystem II (PSII) and biogenesis of the thylakoid membrane
The structure of the oxygen-evolving cyanobacterial PSII complex is now known to atomic resolution. One of our long-term goals is to understand how PSII is assembled from its component parts and how it is repaired following damage. Current models suggest that PSII is assembled in a step-wise manner and requires the participation of accessory factors not found in the final active complex. We are dissecting PSII assembly using an integrated cell biology approach that involves isolation and characterisation of assembly complexes, determination of the structures of PSII assembly and accessory factors and elucidation of their physiological role through analysis of mutants. This work is done in close collaboration with James Murray (Imperial College) and Josef Komenda (Institute of Microbiology, Trebon, Czech Republic). Studies on the biogenesis of the thylakoid membrane are being done in collaboration with Luning Liu (University of Liverpool).
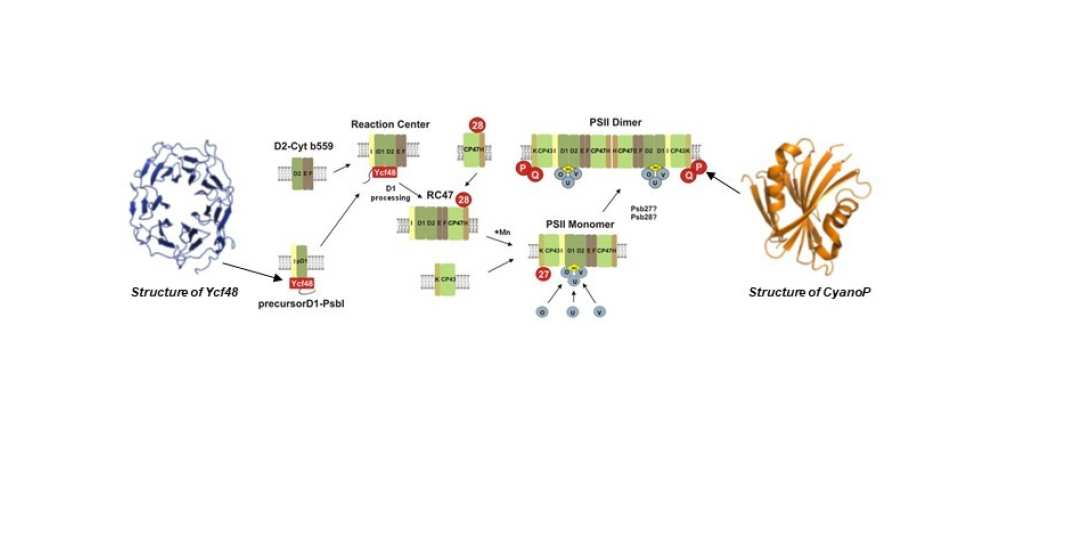
Figure 2: Current model for the step-wise assembly of PSII in cyanobacteria
PSII is a weak link in photosynthesis and prone to damage by light (so-called ‘photoinhibition’), which leads to loss of crop productivity in the field. PSII activity is maintained through the operation of a repair cycle to selectively replace the damaged protein subunit, predominantly the D1 subunit, by a newly synthesised copy. We have identified the FtsH family of proteases as playing a major role in this process and are studying the structures and dynamics of the FtsH complexes within the thylakoid membrane. The role of PSII phosphorylation in PSII repair in chloroplasts is being studied by creating tobacco mutants lacking specific PSII phosphorylation sites, done in collaboration with Conrad Mullineaux and Alexander Ruban at QMUL and Tracy Lawson at the University of Essex.
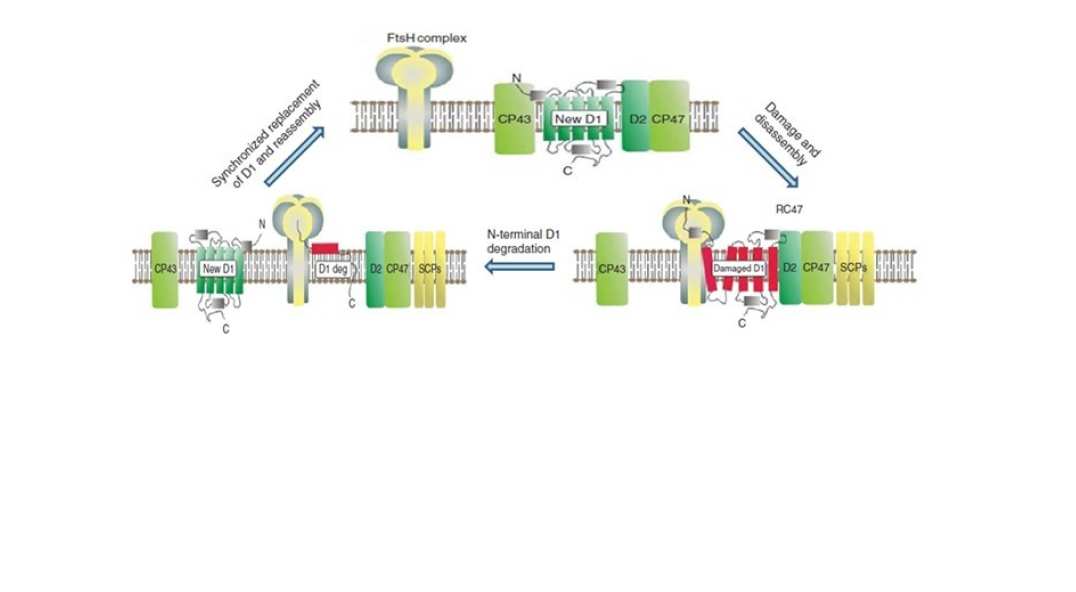
Figure 3: Model for FtsH-mediated degradation of damaged D1 subunit during PSII repair
3) algal and cyanobacterial synthetic biology
We are exploring the use of cyanobacteria and algae to produce high-value products, with particular focus on terpenoids, which are a diverse class of natural products with important uses in the food, health and industrial biotechnology sectors. The advantage of these organisms is that they use solar energy to drive the process and at the same time capture CO2 from the atmosphere. An integrated cell biology approach is being taken to re-engineer metabolism and redirect photosynthetic electron flow; this involves both modelling of metabolism and construction of new strains and synthetic biology tools and involves collaborations with Klaus Hellgardt (Dept of Chemical Engineering), with Sierin Lim at Nanyang Technological University in Singapore and with Patrik Jones at Imperial.
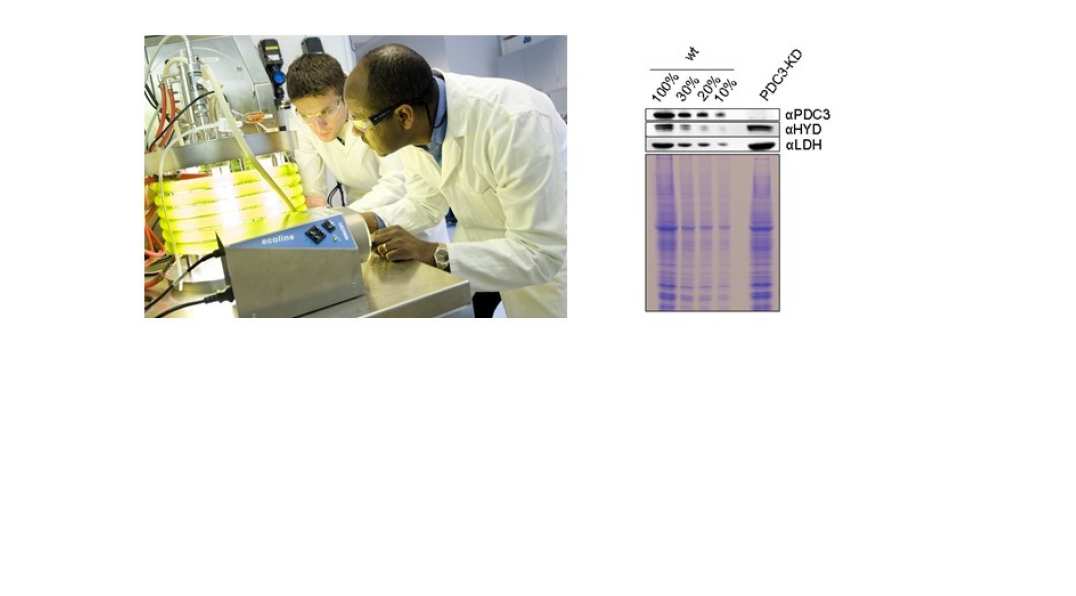
Figure 4: Algal photobioreactor in the Hellgardt Lab (left panel) and creation of knock-down mutants in Chlamydomonas reinhardtii (right panel)
4) Expression of foreign proteins in the chloroplast
We are also using chloroplast transformation technology to express proteins in higher plants as an alternative means for the production of high-value pharmaceutical and commercial proteins. Traditionally transgenic plants have been created by the transformation of the nucleus. However biolistic transformation of the plastid genome offers several advantages. We are currently investigating the practicality of using the chloroplast as a means of expressing a range of foreign proteins. Part of this activity includes the development of bioreactors for the cost-effective production of protein and natural products under totally contained conditions, done in collaboration with Alkion BioInnovations .
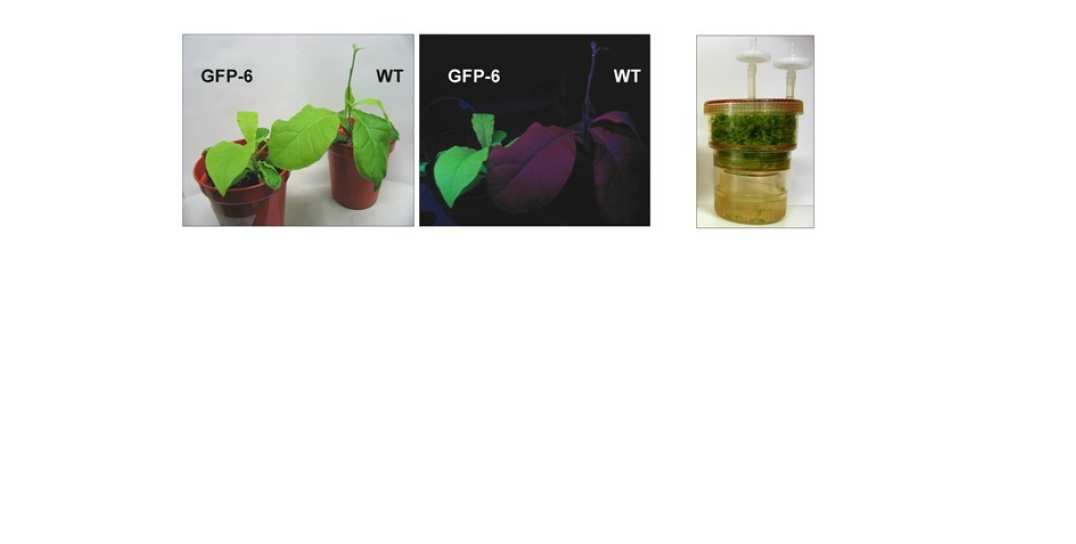
Figure 5: Expression of Green Fluorescent Protein (GFP) in tobacco chloroplasts (left and middle panels) and development of novel bioreactor for production of plant biomass under contained conditions (right panel)
Funding
BBSRC
Guest Lectures
Strategies for enhancing the light reactions of photosynthesis, The 1st Summit Forum on Microalgae and Carbon Neutrality, Nanchang University, China, 2023
Structural insights into photosystem II assembly and repair, Nordic Photosynthesis Congress/Nordic Algae Symposium, Umeå University, Sweden, 2023
Structural insights into photosystem II assembly and repair, International Symposium on photosynthesis and chloroplast regulation, Kobe, Japan, 2022
Structural insights into early and late-stage photosystem II assembly intermediates, Satellite Meeting on Photosystem II Water Oxidation, 18th International Congress on Photosynthesis, Dunedin, New Zealand, 2022
Enhancing photosynthesis: the light reactions, 10th International CeBiTec Research Conference, Bielefeld, Germany, 2021
Domesticating cyanobacteria for the sustainable production of chemicals, 1st International Conference on Biodiversity, Surabaya, East Java, Indonesia, 2019
Strategies for enhancing the light reactions of photosynthesis, Huazhong Agricultural University, Wuhan, China, 2019
‘Enhancing photosynthesis and future trends’, Linnean Society of London, Central London, 2019
‘Repair and the evolution of the photosystem II complex of oxygenic photosynthesis’, 1st Asia-Oceania International Congress on Photosynthesis, Beijing, China, 2018
‘Repair and the evolution of photosystem II’, 5th International Workshop on Solar Energy for Sustainability, Institute of Advanced Studies, Nanyang Technological University, Singapore., 2018
Research Staff
Taunt,H
Research Student Supervision
Cheuk,A, Cyanobacterial ATP synthase (Thomas Meier, co-supervisor)
Ford,A, Developing novel BPV devices
Qi,M, Producing chlorophyll f in chloroplasts
Riaz,S, Role of PSII phosphorylation
Zhao,Z, Structural studies on photosynthetic complexes

