Research | Who we are | Opportunities | Publications | Gallery | Contact
Light-Activated Metal-dependent Protein Degradation (LAMP-D): A Heterobifunctional Ruthenium(II) Photosensitizer Targeting New Delhi Metallo-β-lactamase 1
Lars Stevens-Cullinane, Thomas W Rees, Calum Evans, Po-Yu Ho, Mika Kintzel, Yew Mun Yip, Ruoning Jia, Jonathan Bailey, Eleanor Clifford, Ruqaiya Alam, Sarah Maslen, Stephane Mouilleron, Adrien Pasquier. ChemRxiv, 2025, DOI: 10.26434/chemrxiv-2025-jx9jt
Characterization of a semiquinone radical bound in the active site of respiratory complex I by hyperfine EPR spectroscopy
Eleanor Clifford, John Wright, Alberto Collauto, Judy Hirst, Maxie Roessler. ChemRxiv, 2025, DOI: 10.26434/chemrxiv-2025-br3z5
Revealing the Nature of Non-Covalent Interactions in Ionic Liquids by Combined Pulse EPR and 19F NMR Spectroscopy
Dr. Ciarán J. Rogers, Dr. Spyridon Koutsoukos, Jun. Prof. Jana Eisermann, Dr. Luke Wylie, Gavin J. Smith, Prof. Tom Welton, Dr. Maxie M. Roessler. Angew. Chem. Int. Ed., 2025, DOI: 10.1002/anie.202504882

Versatile high-sensitivity EPR using superconducting spiral microresonators
Gediminas Usevicius, Mantas Simenas, Blaise L. Geoghegan, Oscar W. Kennedy, Ignas Pocius, Patrick Hogan, Ana Villanueva Ruiz de Temino, Jean-Baptiste Verstraete, Paulina Verbaityte, Angeliki Chatziathanasiou, G. Antilen Jacob, Mindaugas Kamarauskas, Marius Treideris, Paulius Gecys, Joseph Alexander, Vidmantas Kalendra, Juras Banys, Maxie M. Roessler, John J.L. Morton . ChemRxiv, 2025, DOI: 10.26434/chemrxiv-2025-c3qv1
Introducing Bonnie Murphy, Ville Kaila, Maxie Roessler, Volha Chukhutsina, Alisia Fadini, Sonya Hanson, Filipe Maia, and Kirill Kovalev
Bonnie J. Murphy , Ville R.I. Kaila, Maxie M. Roessler, Volha Chukhutsina, Alisia Fadini, Sonya M. Hanson, Filipe R.N.C. Maia, Kirill Kovalev. 2025. Voices, 33(5), 829-835. Link to article.
The [2Fe-2S] cluster of mitochondrial outer membrane protein mitoNEET has an O2-regulated nitric oxide access tunnel
Thao Nghi Hoang, Meritxell Wu-Lu, Alberto Collauto, Peter-Leon Hagedoorn, Madalina Alexandru, Maike Henschel, Shahram Kordasti, Maria Andrea Mroginski, Maxie M. Roessler, Kourosh H. Ebrahimi. FEBS Letters, 2025, DOI: 10.1002/1873-3468.15097
Conjugated molecular wires on indium-tin oxide: investigation of the electron transfer mechanism and application in tin perovskite solar cells
Fang Fang, Ang Li, Blaise L. Geoghegan, Yunfei Dang, Troy L. Bennett, Amanz Azaden, Francesco Vanin, Adam J. Sills, Nicholas J. Long, Saif A. Haque* and Maxie M. Roessler*. ChemRxiv, 2024, DOI: 10.26434/chemrxiv-2024-gqwk5
Cover Feature: The Effect of Reactive Oxygen Species on Respiratory Complex I Activity in Liposomes
Jana Eisermann, Yuxin Liang, John Joseph Wright, Eleanor Clifford, James D.E.T. Wilton-Ely, Marina Kuimova, Maxie Roessler, Chem. Eur. J., 2024, DOI: 10.1002/chem.202402035
Mixed Valence {Ni2+Ni1+} Clusters as Models of Acetyl Coenzyme A Synthase Intermediates
Daniel W. N. Wilson, Benedict C. Thompson, Alberto Collauto, Reagan X. Hooper, Caroline E. Knapp, Maxie M. Roessler and Rebecca A. Musgrave*, JACS, 2024, DOI: 10.1021/jacs.4c06241

A Paramagnetic Nickel–Zinc Hydride Complex
Marina Perez-Jimenez, Blaise Geoghegan, Alberto Collauto, Maxie Roessler, Mark Crimmin, Angew. Chem., 2024. DOI: 10.1002/anie.202411828
Synthesis and Characterization of Co (II) Substituted Keggin‐Type Polyoxometalates as Novel Catalysts for the Hydroformylation of 1‐Hexene in a Thermomorphic Solvent System
Jan‐Christian Raabe, Lea Hombach, Maximilian J Poller, Alberto Collauto, Maxie M Roessler, Andreas Vorholt, Anna Katharina Beine, Jakob Albert, Chem. Cat. Chem., 2024, DOI: 10.1002/cctc.202400395
-Substituted-KegginâType-Polyoxometalates-as-Novel-Catalysts-for-the-Hydroformylation-of-1âHexene-in-a-Thermomorphic-Solvent-System.png)
Operando film-electrochemical EPR spectroscopy tracks radical intermediates in surface-immobilized catalysts
Maryam Seif-Eddine, Samuel J. Cobb, Yunfei Dang, Kaltum Abdiaziz, Mark A. Bajada, Erwin Reisner & Maxie M. Roessler, Nat. Chem., 2024, DOI: 10.1038/s41557-024-01450-y

Film-electrochemical EPR spectroscopy to investigate electron transfer in membrane proteins in their native environment
Davide Facchetti, Yunfei Dang, Maryam Seif-Eddinea, Blaise L. Geoghegan and Maxie M. Roessler*, Chem. Commun., 2024, DOI: 10.1039/D4CC04013A

Heterobimetallic 3d-4f complexes supported by a Schiff-base tripodal ligand
Rebecca A Musgrave , Benedict C Thompson , Daniel Graycon , Denny Hebron , Till Neumann , Maxie Roessler , Alberto Collauto and Daniel Wilson, Dalton Transactions, 2024, DOI: 10.1039/D3DT03760F
Understanding the effects of targeted modifications on the 1:2 Choline And GEranate structure
Ana Dobre, Spyridon Koutsoukos, Frederik Philippi, Daniel Rauber, Christopher W. M. Kay, Oriele Palumbo, Maxie M. Roessler and Tom Welton*, Phys. Chem. Chem. Phys., 2024, DOI: 10.1039/D3CP05271K
Using light scattering to assess how phospholipid-protein interactions affect complex I functionality in liposomes
Jana Eisermann, John Joseph Wright, James Wilton-Ely, Judy Hirst and Maxie Roessler, RSC chem. biol., 2023, DOI: 10.1039/D2CB00158F

Radical-SAM dependent nucleotide dehydratase (SAND), rectification of the names of an ancient iron-sulfur enzyme using NC-IUBMB recommendations
Yuxuan Ji, Li Wei, Anqi Da, Holger Stark, Peter-Leon Hagedoorn, Simone Ciofi-Baffoni, Sally A. Cowley, Ricardo O. Louro, Smilja Todorovic, Maria Andrea Mroginski, Yvain Nicolet, Maxie M. Roessler, Nick E. Le Brun, Mario Piccioli, William S. James, Wilfred R. Hagen and Kourosh H. Ebrahimi*, Front. Mol. Biosci. 2022, DOI: 10.3389/fmolb.2022.1032220
,-rectification-of-the-names-of-an-ancient-iron-sulfur-enzyme-using-NC-IUBMB-recommendations.jpg)
Bio-Electrocatalytic Conversion of Food Waste to Ethylene via Succinic Acid as the Central Intermediate
Christian M. Pichler, Subhajit Bhattacharjee, Erwin Lam, Lin Su, Alberto Collauto, Maxie M. Roessler, Samuel J. Cobb, Vivek M. Badiani, Motiar Rahaman, and Erwin Reisner*, ACS Catal. 2022, DOI: 10.1021/acscatal.2c02689

Following the evolution of paramagnetic species during catalysis: film-electrochemical EPR spectroscopy
Maryam Seif-Eddine, Kaltum Abdiaziz, Mark Bajada, Erwin Reisner, Maxie M.Roessler, Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2022, DOI: 10.1016/j.bbabio.2022.148742
Controlling and exploiting intrinsic unpaired electrons in metalloproteins.
Katherine H. Richardson, Maryam Seif-Eddine, Adam Sills, Maxie M. Roessler, Methods in Enzymology, 2022, DOI: 10.1016/bs.mie.2022.02.014
Functional basis of electron transport within photosynthetic complex I
Katherine H. Richardson, John J. Wright, Mantas Šimėnas, Jacqueline Thiemann, Ana M. Esteves, Gemma McGuire, William K. Myers, John J. L. Morton, Michael Hippler, Marc M. Nowaczyk, Guy T. Hanke & Maxie M. Roessler, Nature Communications, 2021, DOI: 10.1038/s41467-021-25527-1

Rotaxane CoII Complexes as Field‐Induced Single‐Ion Magnets
Martina Cirulli, Enrico Salvadori, Zhi‐Hui Zhang, Michael Dommett, Floriana Tuna, Heiko Bamberger, James EM Lewis, Amanpreet Kaur, Graham J Tizzard, Joris Van Slageren, Rachel Crespo‐Otero, Stephen M Goldup, Maxie M Roessler, Angewandte Chemie International Edition, 2021. DOI: 10.1002/anie.202103596
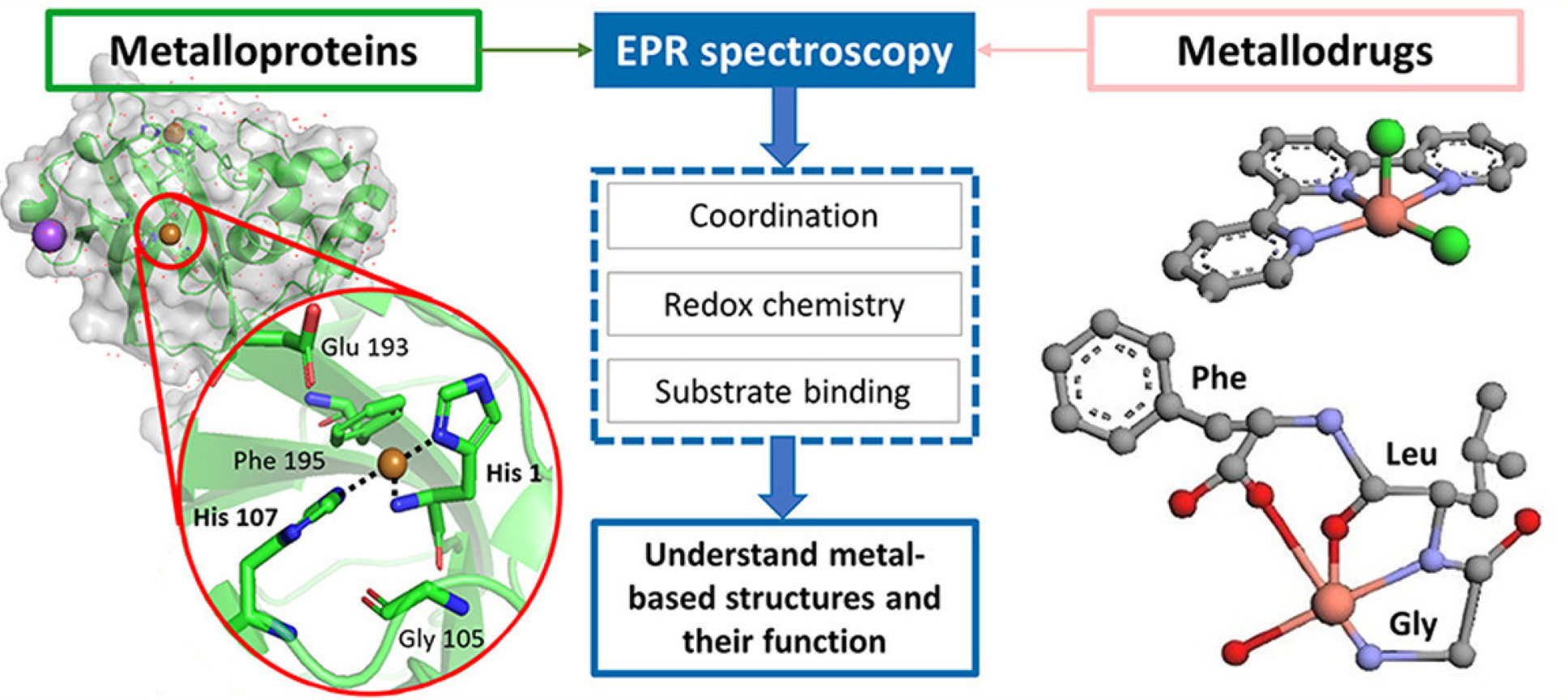
Insights into metalloproteins and metallodrugs from electron paramagnetic resonance spectroscopy
Jana Eisermann, Maryam Seif-Eddine, Maxie M.Roessler, Current Opinion in Chemical Biology, Volume 61, 2021, Pages 114-122. DOI: 10.1016/j.cbpa.2020.11.005
Functional group introduction and aromatic unit variation in a set of π‑conjugated macrocycles: revealing the central role of local and global aromaticity
Martina Rimmele, Wojciech Nogala, Maryam Seif-Eddine, Maxie Roessler, Martin Heeney, Felix Plasser, Florian Glöcklhofer, Organic Chemistry Frontiers, 2021. DOI: 10.1039/D1QO00901J

A conserved arginine residue is critical for stabilizing the N2 FeS cluster in mitochondrial complex I
Mikhail A Hameedi, Daniel N Grba, Katherine H Richardson, Andrwe JY Jones, Wei Song, Maxie M Roessler, John J Wright, Judy Hirst, Journal of Biological Chemistry, volume 296, 100474, 2021. DOI: 10.1016/j.jbc.2021.100474
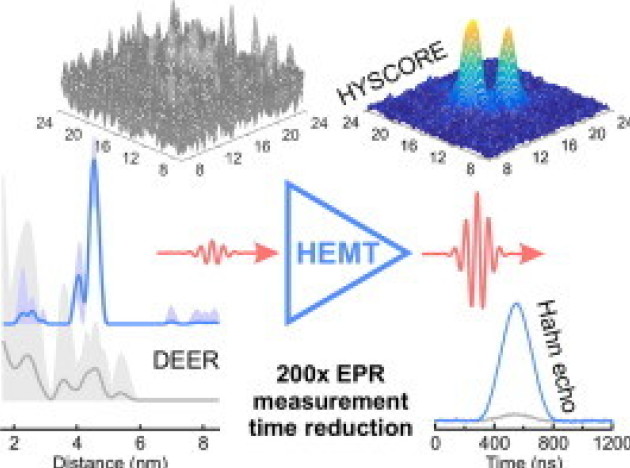
A sensitivity leap for X-band EPR using a probehead with a cryogenic preamplifier
Mantas Šimėnas, James O’Sullivan, Christoph W Zollitsch, Oscar Kennedy, Maryam Seif-Eddine, Irina Ritsch, Miriam Hülsmann, Mian Qi, Adelheid Godt, Maxie M Roessler, Gunnar Jeschke, John JL Morton, Journal of Magnetic Resonance, Volume 322, 2020, Article 106876. DOI: 10.1016/j.jmr.2020.106876
Using a chimeric respiratory chain and EPR spectroscopy to determine the origin of semiquinone species previously assigned to mitochondrial complex I
John J Wright, Justin G Fedor, Judy Hirst, Maxie M Roessler, 2020, BMC Biol 18, 54, 2020. DOI: 10.1186/s12915-020-00768-6
Structure of inhibitor-bound mammalian complex I
Hannah R Bridges, Justin G Fedor, James N Blaza, Andrea Di Luca, Alexander Jussupow, Owen D Jarman, John J Wright, Ahmed-Noor A Agip, Ana P Gamiz-Hernandez, Maxie M Roessler, Ville RI Kaila, Judy Hirst, 2020, Nat Commun 11, 5261 (2020). DOI: 10.1038/s41467-020-18950-3
A Precious‐Metal‐Free Hybrid Electrolyzer for Alcohol Oxidation Coupled to CO2‐to‐Syngas Conversion
Mark A Bajada, Souvik Roy, Julien Warnan, Kaltum Abdiaziz, Andreas Wagner, Maxie M Roessler, Erwin Reisner, 2020, Angewandte Chemie, Vol: 132, Pages: 15763-15771, ISSN: 0044-8249. DOI: 10.1002/anie.202002680
Manipulating the Optical Properties of Carbon Dots by Fine‐Tuning their Structural Features
Hui Luo, Nikolaos Papaioannou, Enrico Salvadori, Maxie M Roessler, Gereon Ploenes, Ernst RH van Eck, Liviu C Tanase, Jingyu Feng, Yiwei Sun, Yan Yang, Mohsen Danaie, Ana Belen Jorge, Andrei Sapelkin, James Durrant, Stoichko D Dimitrov, Maria‐Magdalena Titirici, 2019, ChemSusChem, 12 (19), 4432-4441. DOI: 10.1002/cssc.201901795
Defining optimal electron transfer partners for light-driven cytochrome P450 reactions
Silas Busck Mellor, Marcos Hamborg Vinde, Agnieszka Zygadlo Nielsen, Guy Thomas Hanke, Kaltum Abdiaziz, Maxie M Roessler, Meike Burow, Mohammed Saddik Motawia, Birger Lindberg Møller, Poul Erik Jensen, 2019, Metabolic engineering, 55, 33-43. DOI 10.1016/j.ymben.2019.05.003
Protein film electrochemical EPR spectroscopy as a technique to investigate redox reactions in biomolecules
K. Abdiaziz, E. Salvadori, K. P. Sokol, E. Reisner, M. M. Roessler, 2019, Chem. Commun., 2019,55, 8840-8843, DOI: 10.1039/C9CC03212F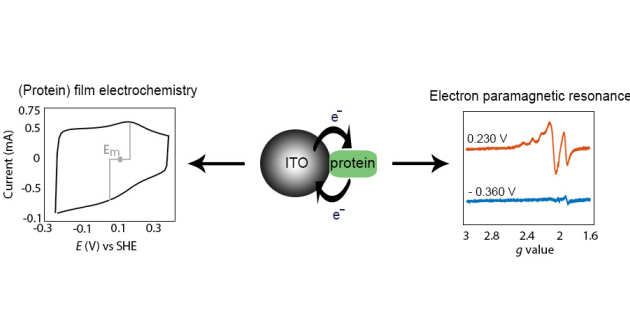
Rotaxane-Based Transition Metal Complexes: Effect of the Mechanical Bond on Structure and Electronic Properties
M. Cirulli, A. Kaur, J.E.M. Lewis, Z. Zhang, J.A. Kitchen, S.M. Goldup*, M.M. Roessler* (2018), J. Am. Chem. Soc., DOI: 10.1021/jacs.8b09715
X-ray structural, functional and computational studies of the O 2-sensitive E. coli hydrogenase-1 C19G variant reveal an unusual [4Fe-4S] cluster
A. Volbeda, J.-M. Mouesca, C. Darnault, M.M. Roessler, A. Parkin, F. A. Armstrong, J. C. Fontecilla-Camps* (2018), Chemical Communications, 54, 7175-7178. DOI: 10.1039/C8CC02896F
![X-ray structural, functional and computational studies of the O 2-sensitive E. coli hydrogenase-1 C19G variant reveal an unusual [4Fe-4S] cluster](/media/migration/research-groups/ChemComm_Volbeda--tojpeg_1557767539333_x2.jpg)
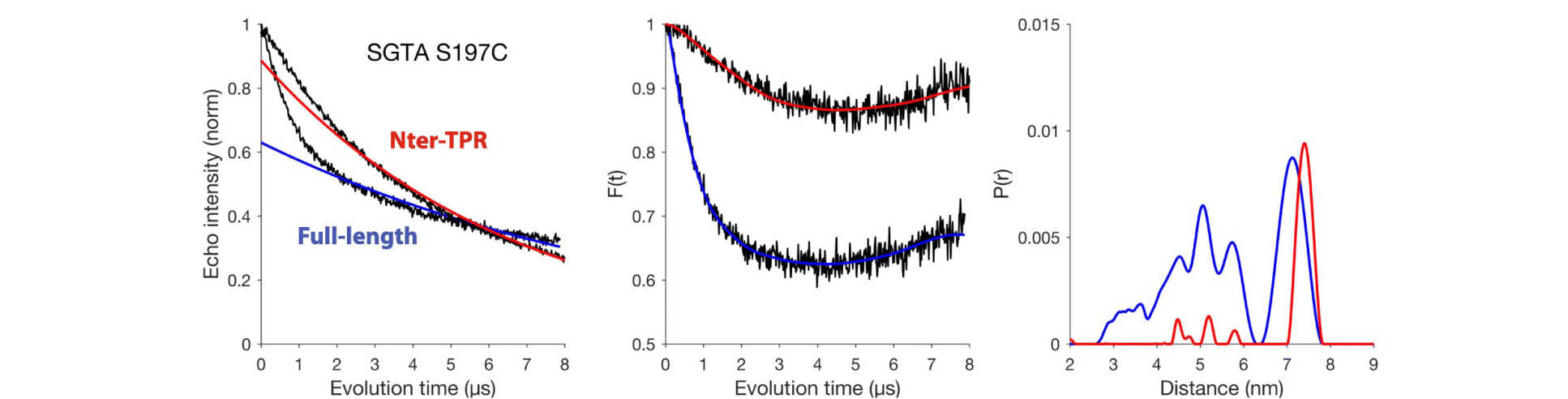
Structural complexity of the co-chaperone SGTA: a conserved C-terminal region is implicated in dimerization and substrate quality control
S. Martínez-Lumbreras, E. M. Krysztofinska, A. Thapaliya, A. Spilotros, D. Matak-Vinkovic, E. Salvadori, P. Roboti, Y. Nyathi, J. H. Muench, M. M. Roessler, D.I. Svergun, S. High , R. L. Isaacson* (2018), BMC Biology, 16, 76. DOI: 10.1186/s12915-018-0542-3
Principles and Applications of EPR Spectroscopy in the chemical sciences
M. M. Roessler* and E. Salvadori* (2018), Chemical Society Reviews, 47 (8), 2534-2553. DOI: 10.1039/C6CS00565A
Using EPR Hyperfine Spectroscopy to define the Proton-Coupled Electron Transfer Reaction at Fe-S cluster N2 in Respiratory Complex
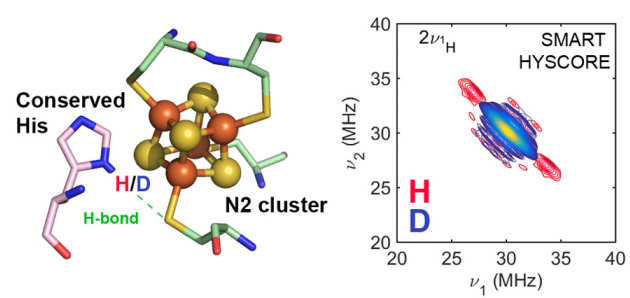 N. le Breton, J. J. Wright, A.J.Y.J. Jones, E. Salvadori, H. R. Bridges, J. Hirst, M. M. Roessler* (2017), J. Am. Chem. Soc., 139 (45), 16319-16326. DOI: 10.1021/jacs.7b09261 selected as Spotlight Article
N. le Breton, J. J. Wright, A.J.Y.J. Jones, E. Salvadori, H. R. Bridges, J. Hirst, M. M. Roessler* (2017), J. Am. Chem. Soc., 139 (45), 16319-16326. DOI: 10.1021/jacs.7b09261 selected as Spotlight Article
Re-tuning the Catalytic Bias and Overpotential of a [NiFe]-hydrogenase via a Single Amino Acid Exchange at the Electron Entry/exit site
H. Adamson, M. Robinson, J. J. Wright, L. A. Flanagan, J. Walton, D. Elton, D. J. Gavaghan, A. M. Bond, M.M. Roessler, A. Parkin (2017), J. Am. Chem. Soc., 139 (31), pp 10677-1068. DOI: 10.1021/jacs.7b03611
![Re-tuning the Catalytic Bias and Overpotential of a [NiFe]-hydrogenase via a Single Amino Acid Exchange at the Electron Entry/exit site](/media/migration/research-groups/ja-2017-03611w_0005--tojpeg_1557928025318_x2.jpg)
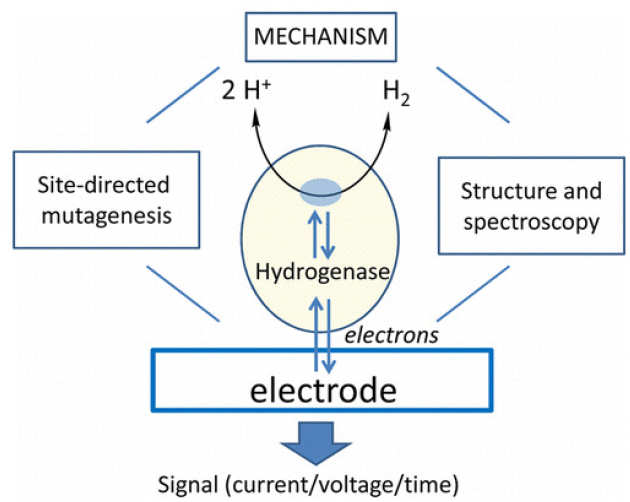
Guiding Principles of Hydrogenase Catalysis Instigated and Clarified by Protein Film Electrochemistry
F. A. Armstrong*, R. M. Evans, S. V. Hexter, B. J. Murphy, M. M. Roessler, P. Wulff (2016), Acc. Chem. Res. 49 (5), pp 884-892. DOI: 10.1021/acs.accounts.6b00027
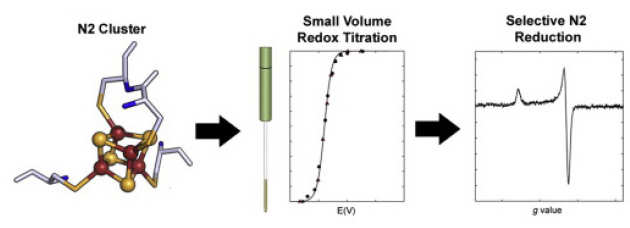
Small-volume potentiometric titrations: EPR investigations of Fe-S cluster N2 in mitochondrial complex I
J. J. Wright, E. Salvadori, H. R. Bridges, J. Hirst & M. M. Roessler* (2016), J. Inorg. Biochem. 162, pp 201-206. DOI: 10.1016/j.jinorgbio.2016.04.025
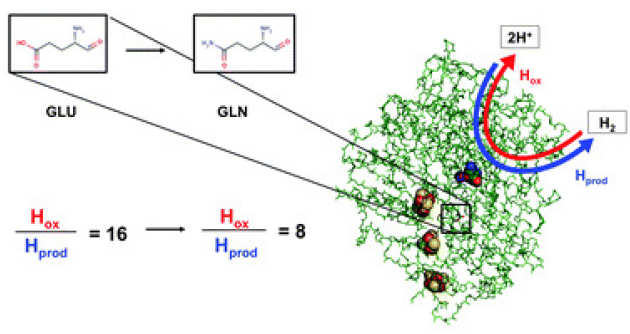
Re-engineering a NiFe hydrogenase to increase the H2 production bias while maintaining native levels of O2 tolerance
L. A. Flanagan, J. J. Wright, M. M. Roessler, J.W. Moir & A. Parkin (2016), Chem. Commun. 52, pp 9133-9136. DOI: 10.1039/C6CC00515B
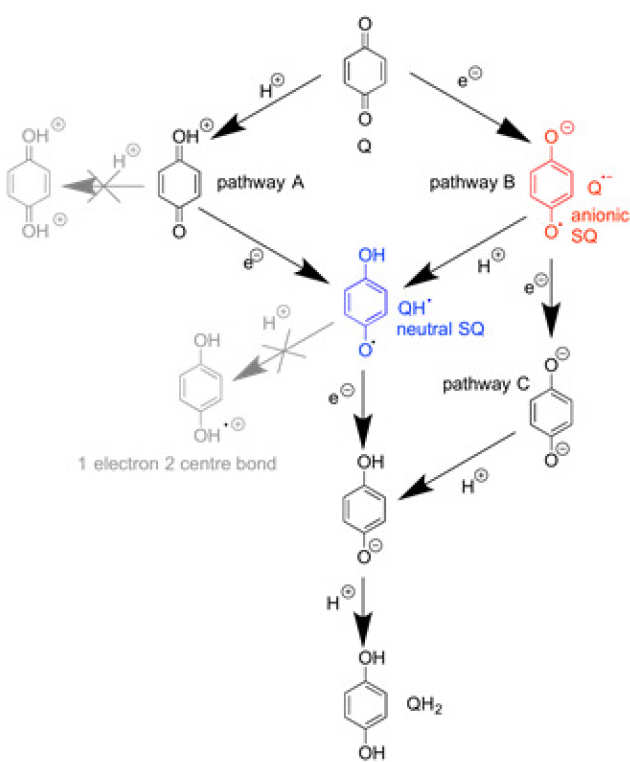
Energy conversion, redox catalysis and generation of reactive oxygen species by complex I
J. Hirst* & M. M. Roessler* (2016), BBA Bioenergetics. 1857 (7), pp 872-883. DOI: 10.1016/j.bbabio.2015.12.009
Discovery of Dark pH-dependent H+ Migration in a [NiFe]-hydrogenase and Its Mechanistic Relevance: Mobilising the Hydrido Ligand of the Ni-C Intermediate
B.J. Murphy,R. Hidalgo, M.M. Roessler, R.M. Evans, P.A. Ash, W.K. Myers, K.A. Vincent, and F.A. Armstrong (2015), J. Am. Chem. Soc. 137 (26), pp 8484-8489. DOI: 10.1021/jacs.5b03182
!['Discovery of Dark pH-dependent H+ Migration in a [NiFe]-hydrogenase and Its Mechanistic Relevance: Mobilising the Hydrido Ligand of the Ni-C Intermediate](/media/migration/research-groups/ja-2015-03182x_0007--tojpeg_1557929549590_x2.jpg)
Principles of Sustained Enzymatic Hydrogen Oxidation in the Presence of Oxygen – The Crucial Influence of High Potential Fe–S Clusters in the Electron Relay of [NiFe]-Hydrogenases
R.M. Evans, A. Parkin, M.M. Roessler, B.J. Murphy, H. Adamson, M.J. Lukey, F. Sargent, A. Volbeda, J.C. Fontecilla-Camps, and F.A. Armstrong (2013), J. Am. Chem. Soc. 135 (7), pp 2694–2707. DOI: 10.1021/ja311055d
![Principles of Sustained Enzymatic Hydrogen Oxidation in the Presence of Oxygen – The Crucial Influence of High Potential Fe–S Clusters in the Electron Relay of [NiFe]-Hydrogenases](/media/migration/research-groups/ja-2012-11055d_0010--tojpeg_1557929657615_x2.jpg)
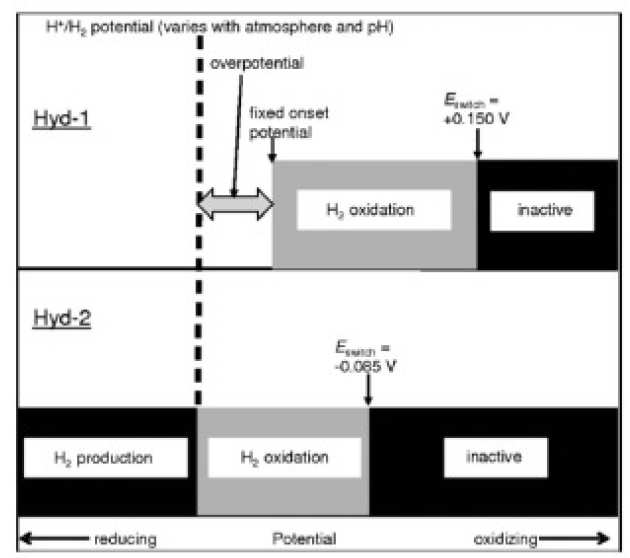
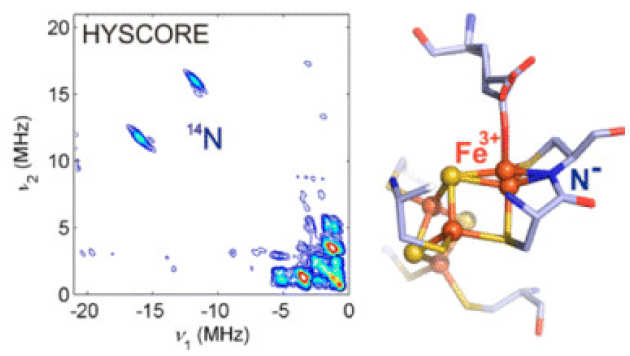
EPR Spectroscopic Studies of the Fe–S Clusters in the O2-Tolerant [NiFe]-Hydrogenase Hyd-1 from Escherichia coli and Characterization of the Unique [4Fe–3S] Cluster by HYSCORE
M.M. Roessler, R.M. Evans, R.A. Davies, J. Harmer, and F.A. Armstrong (2012), J. Am. Chem. Soc. 134(37), pp 15581–15594. DOI: 10.1021/ja307117y
X-ray crystallographic and computational studies of the O2-tolerant [NiFe]-hydrogenase 1 from Escherichia coli
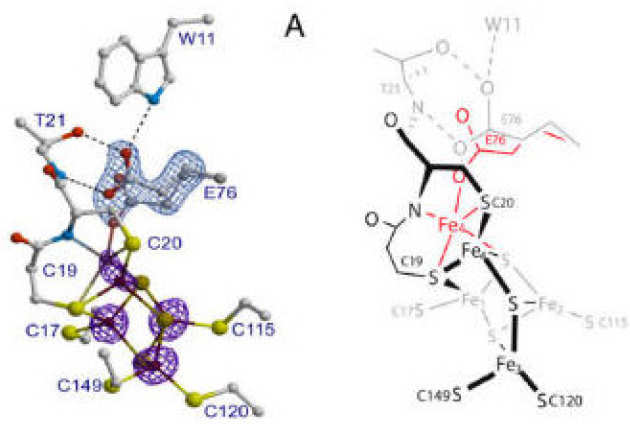
A. Volbeda, P. Amara, C. Darnault, J.-M. Mouesca, A. Parkin, M.M. Roessler, F.A. Armstrong, J. C Fontecilla-Camps (2012), Proceedings of the National Academy of Sciences 109 (14), pp 5305–5310. DOI: 10.1073/pnas.1119806109
How Salmonella oxidises H2 under aerobic conditions
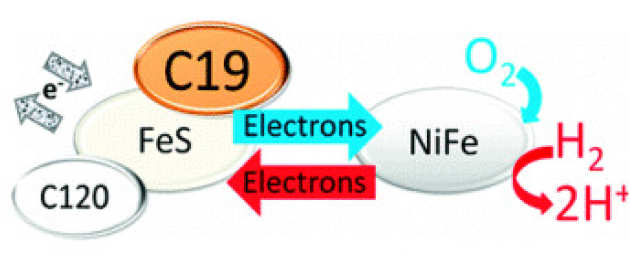
A. Parkin, L. Bowman, M.M. Roessler, R.A. Davies, T. Palmer, F.A. Armstrong, and F. Sargent (2012), FEBS letters 586 (5), 536-544. DOI: 10.1016/j.febslet.2011.07.044
Oxygen-Tolerant [NiFe]-Hydrogenases: The Individual and Collective Importance of Supernumerary Cysteines at the Proximal Fe-S Cluster
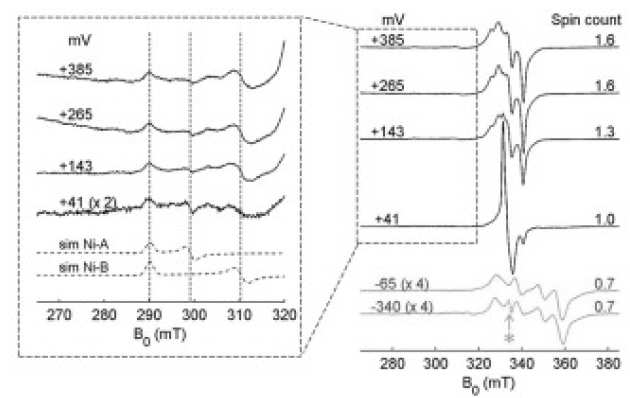
M.J. Lukey, M.M. Roessler, A. Parkin, R.M. Evans, R.A. Davies, O. Lenz, B. Friedrich, F. Sargent, and F.A. Armstrong (2011), J. Am. Chem. Soc. 133 (42), pp 16881–16892. DOI: 10.1021/ja205393w
Theoretical and experimental investigation of surface-confined two-center metalloproteins by large-amplitude Fourier transformed ac voltammetry
C.Y. Lee, G.P. Stevenson, A. Parkin, M.M. Roessler, R.E. Baker, K. Gillow, D.J. Gavaghan, F.A. Armstrong, and A.M. Bond (2011), Journal of Electroanalytical Chemistry 656 (1), 293-303.
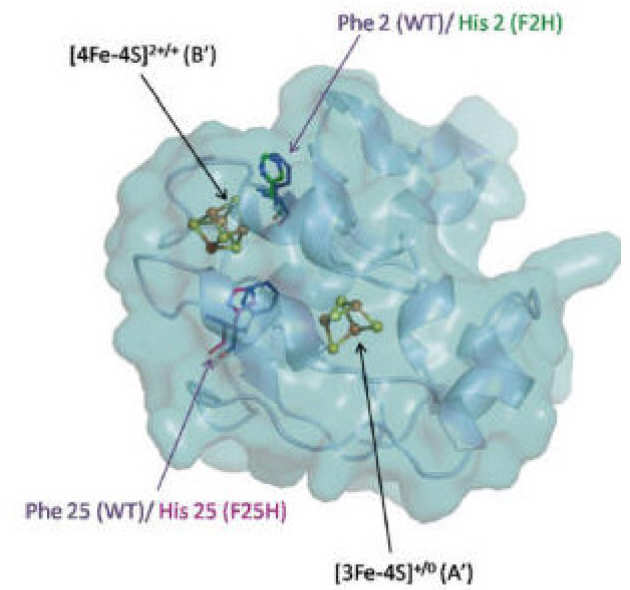
Direct assignment of EPR spectra to structurally defined iron-sulfur clusters in complex I by double electron–electron resonance
M.M. Roessler, M.S. King, A.J. Robinson, F.A. Armstrong, J. Harmer, and J. Hirst (2010), Proceedings of the National Academy of Sciences 107 (5), 1930-1935. DOI: 10.1073/pnas.0908050107
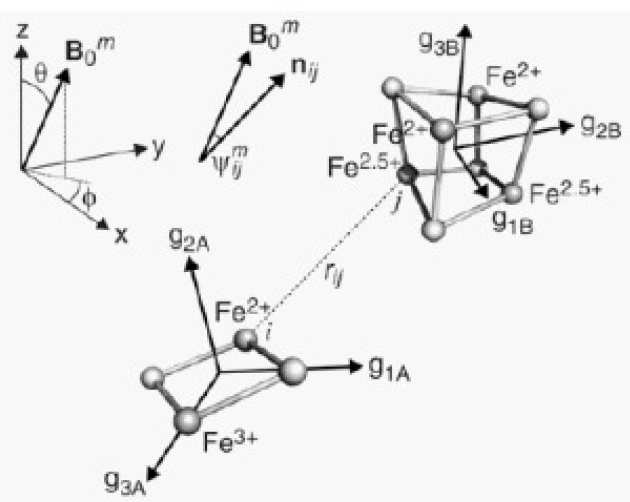
How Escherichia coli is equipped to oxidize hydrogen under different redox conditions
M.J. Lukey, A. Parkin, M.M. Roessler, B.J. Murphy, J. Harmer, T. Palmer, F. Sargent, and F.A. Armstrong (2010), Journal of Biological Chemistry 285 (6), 3928-3938. DOI: 10.1074/jbc.M109.067751