Chiral functional materials and devices
Chirality is a fundamental symmetry property; chiral objects, such as chiral molecules, exist as a pair of non-superimposable mirror images. Although chirality in molecular design is routinely considered in biologically focused application areas (such as drug discovery and chemical biology), other areas of scientific development have not considered chirality to be central to their approach. By harnessing the polarisation of photons and the spin of electrons, chirality provides a new approach to many applications, from bioimaging, to encrypted optical communication, to energy-efficient displays. We have advocated the potential of chiral conjugated materials for these next-generation technologies and beyond. We use a range of chiral systems, including chiral conjugated small molecules, polymers, nanomaterials (such as fullerenes) and hybrid organic-inorganic perovskite materials to explore the functional potential of chirality in optoelectronic technologies. Reviews: Chem. Sci. 2021, 12, 8589. DOI; Nature Rev. Chem. 2017, 1, 0045. DOI.
Novel Materials
Chiral light emission
Circularly polarised (CP) OLEDs. CP light is a chiral form of electromagnetic radiation and is central to a large range of current and future display and photonic technologies, including highly efficient displays, optical quantum information processing and communication, and optical spintronics. There is therefore high interest in constructing CP light emitting devices. For more than 10 years, we have been exploring the development of chiral emissive materials for efficient CP organic light emitting diodes (CP-OLEDs).
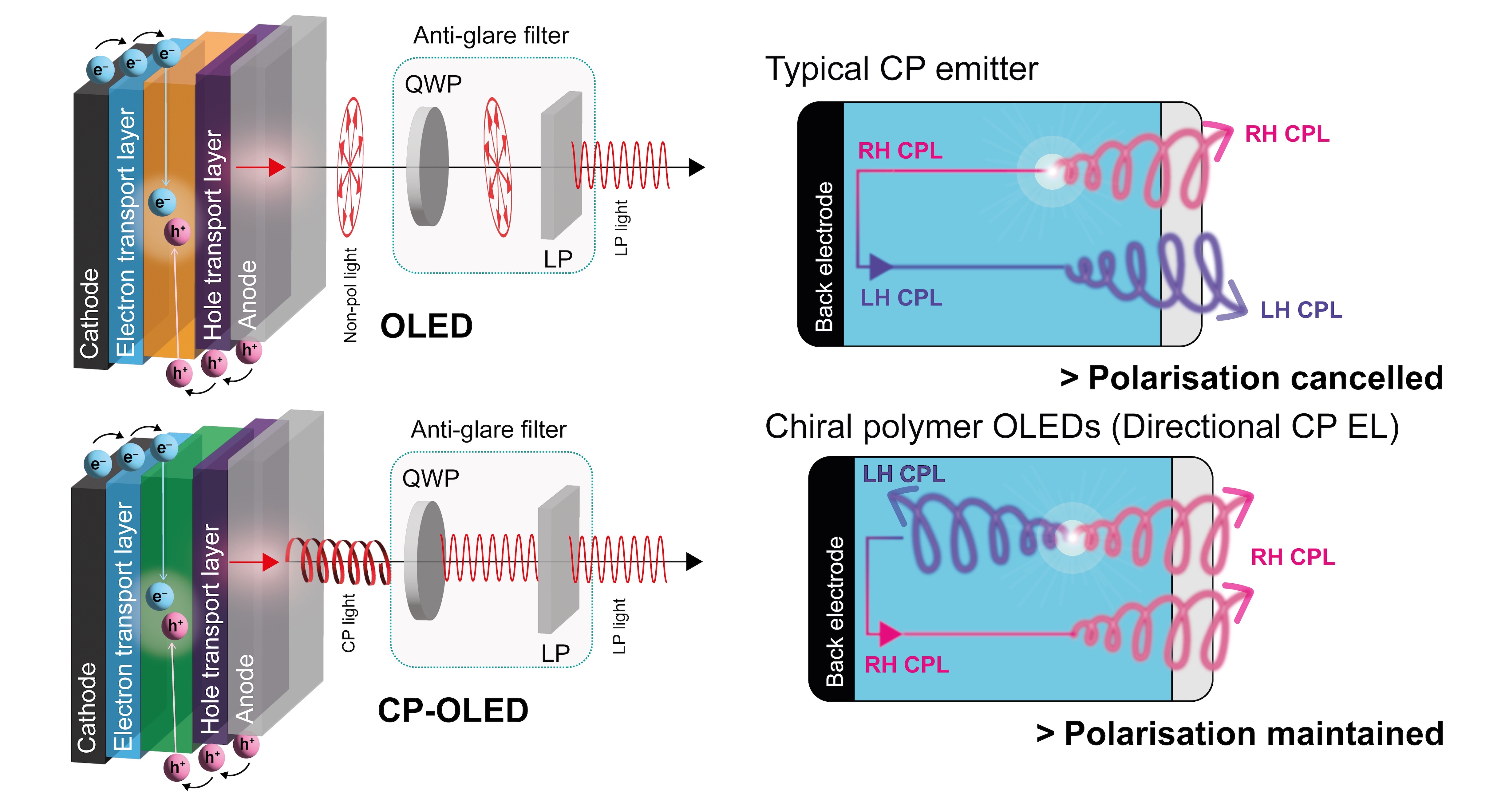
Our most successful approach is based on organic blend materials, which consist of a chiral small molecule and a non-chiral polymer mixture. For example, by combining a conventional light emitting polymer (F8BT) with a small amount of a single handed helically chiral aromatic (a helicene), we have shown that we are able to generate substantial levels of CP photo- and electroluminescence from the polymer. We have optimised this approach to achieve leading levels of circular polarisation from CP-OLEDs and uncovered novel fundamental mechanisms that underpin the chiroptical behaviour of such materials. We have also discovered device-specific ‘anomalous’ mechanisms for the generation of CP electroluminescence in such materials, which are proposed to occur through the orbital polarisation of charge carriers as they propagate through the chiral active layer material. Representative publications: Nature Photon. 2023, 17, 193. DOI; Adv. Opt. Mater. 2021, 2100066. DOI; ACS Appl. Mater. Interfaces 2020, 12, 39471. DOI; ACS Nano 2019, 13, 8099. DOI; Adv. Mater. 2013, 25, 2624. DOI.
CP-FRET to enhance the magnitude of CP emission from chiral small molecules. Strongly dissymmetric CP luminescence from small organic molecules is usually not possible, despite large efforts worldwide. We have described an approach to achieve almost a 1000-fold chiroptical amplification of the CP emission from an emissive helicene when embedded in the chiral phase of a conjugated polymer host. We propose that the amplification arises not simply through a chiral environment effect, but instead due to electrodynamic coupling between the electric and magnetic transition dipoles of the polymer donor and helicene acceptor, and subsequent CP Förster resonance energy transfer (CP-FRET). We believe this approach represents a simple and versatile means to enhance the g-factors of small organic molecules. Further mechanistic studies on the origins of this effect are ongoing. Representative publication: Angew. Chem. Int. Ed. 2021, 60, 222. DOI.
Chiral light detection
The development of CP light-based technologies to their full potential requires the realisation of miniature, integrated devices that can detect the ‘handedness’ of CP light. The circularly selective optical response of chiral functional materials makes them an exciting prospect for direct detection of CP light without the need for external optics. Review: J. Mater. Chem. C, 2022, 10, 10452. DOI.
We have studied the prospect of using a range of chiral materials in a range of device configurations for the direct detection of CP light. Representative examples include:
Small molecule and nanomaterial based CP photoFETs. In 2013 we reported an organic field-effect transistor (OFET) based on enantiomerically pure helicene that can detect and differentiate CP-light, acting as a CP-electrical switch. A highly specific and reversible photo-response to CP light was observed, which is directly related to the handedness of the helicene molecule. More recently, we extended this work using chiral fullerene isomers. Specifically, we separated ten pairs of enantiomers from the 19 structural isomers of a chiral bisfullerene adduct and used them to elucidate important chiroptical relationships in chiral fullerene chromophores. We subsequently demonstrated the application of these materials in a CP photoFET, where the potential to discriminate CPL with a fast light response time and with a very high photocurrent dissymmetry factor was demonstrated. Representative publications: Adv. Mater. 2021, 33, 2004115. DOI; Nature Photon. 2013, 7, 634. DOI.
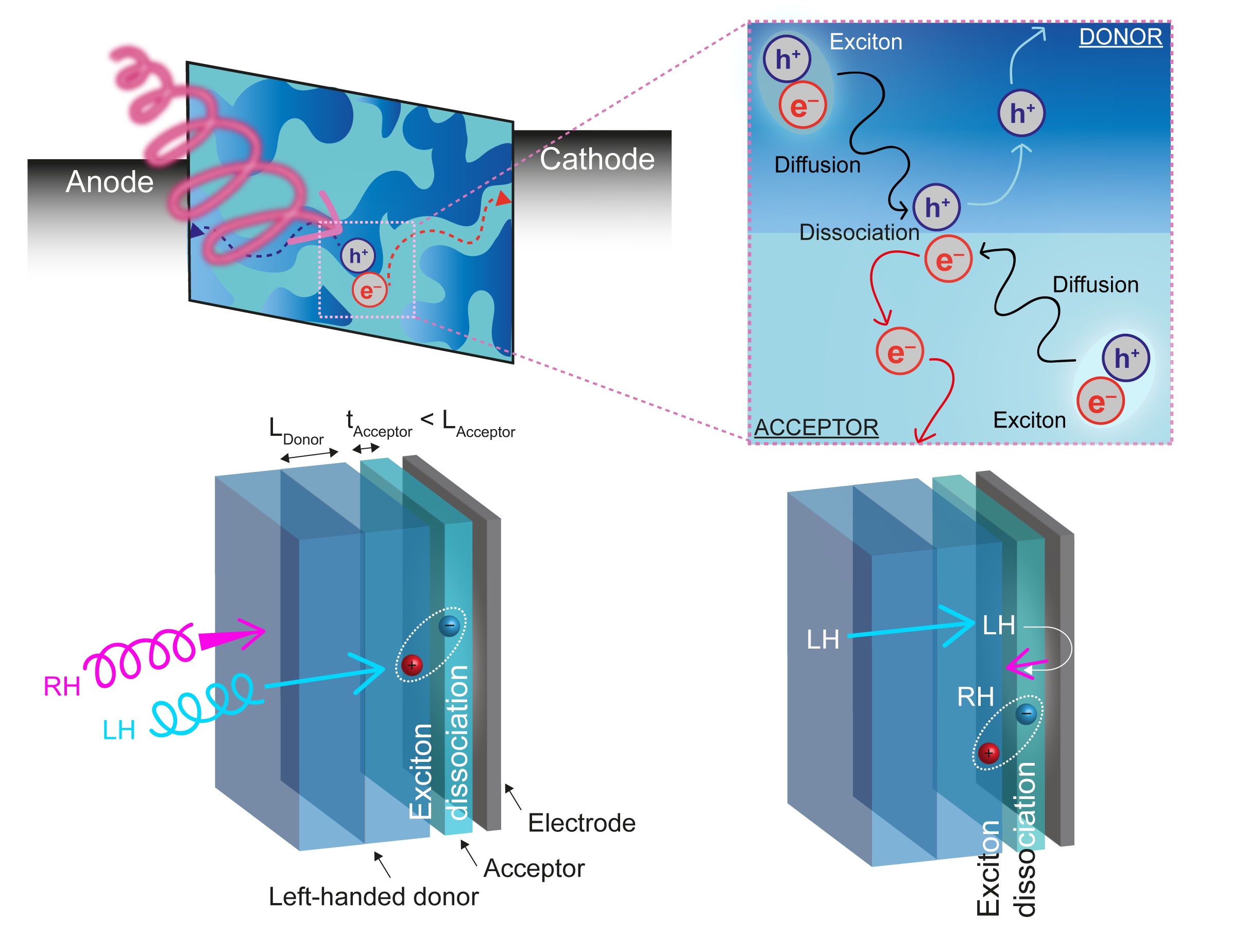
Polymer blend CP photodiodes. Using the same polymer-helicene blend materials that we have found to be so useful for CP-OLEDs, we have prepared bilayer organic photodiodes (CP OPDs) comprising of our chiral blend material as the electron donor layer and an achiral C60 electron acceptor layer. Our devices exhibit high discrimination of CPL (gph), while showing other excellent characteristics including low dark currents and fast response times. We find that the selectivity of the devices to left versus right-handed CP light is sensitive to the thickness of both the chiral donor and achiral acceptor layers and that a trade-off exists between the external quantum efficiency and gph. Representative publication: Adv. Opt. Mater. 2021, 2101044. DOI.
Hybrid perovskite CP photoconductors. Hybrid organic–inorganic perovskites containing chiral organic ligands are an emerging candidate for the active material in CP photodetecting devices, but prior studies suggested there to be a trade-off between the ability of the material to differentially absorb CP light and photocurrent responsivity in chiral perovskite photoconducting devices. We have reported a CP photoconductor device based on quasi two-dimensional (quasi-2D) chiral perovskite films. We find it is possible to generate materials where the circularly selective absorption is comparable in both 2D and quasi-2D films, while the responsivity of the photodetector improves for the latter. Given this, we were able to showcase a CP light photodetector that exhibits both an excellent selectivity for CP light detection and a high responsivity. Representative publication: ACS Nano, 2022, 16, 2682. DOI.
Function via chiral composition
It is well-known that the key to successful and high-performance organic devices is the need to link well-understood characteristics of an isolated molecule (so-called “molecular” properties) into the collective behaviour of multiple units in thin films (“material” properties). When employing a chiral organic semiconducting material, it is possible to use a range of chiral compositions, differing in the proportion of left- and right-handed structures; the most common being a racemate (a 50:50 mixture of left-handed and right-handed molecules) and an enantiopure (single handedness) composition. As the right- and left-handed enantiomers of a given chiral material have identical “molecular” properties, such properties would not be expected to change when comparing an enantiopure composition to a racemate. However, enantiopure and racemic materials have different bulk packing and therefore different bulk material properties. This possibility to exploit chirality to alter the “material” properties without affecting the “molecular” properties is a fascinating concept which we continue to explore.
We have previously shown that organic field-effect transistors (OFETs) constructed from the helicenes can display large differences in charge carrier mobility, together with differences in thin-film photophysics and morphology, solely depending on whether enantiopure or racemic chiral compositions are employed under analogous fabrication conditions.
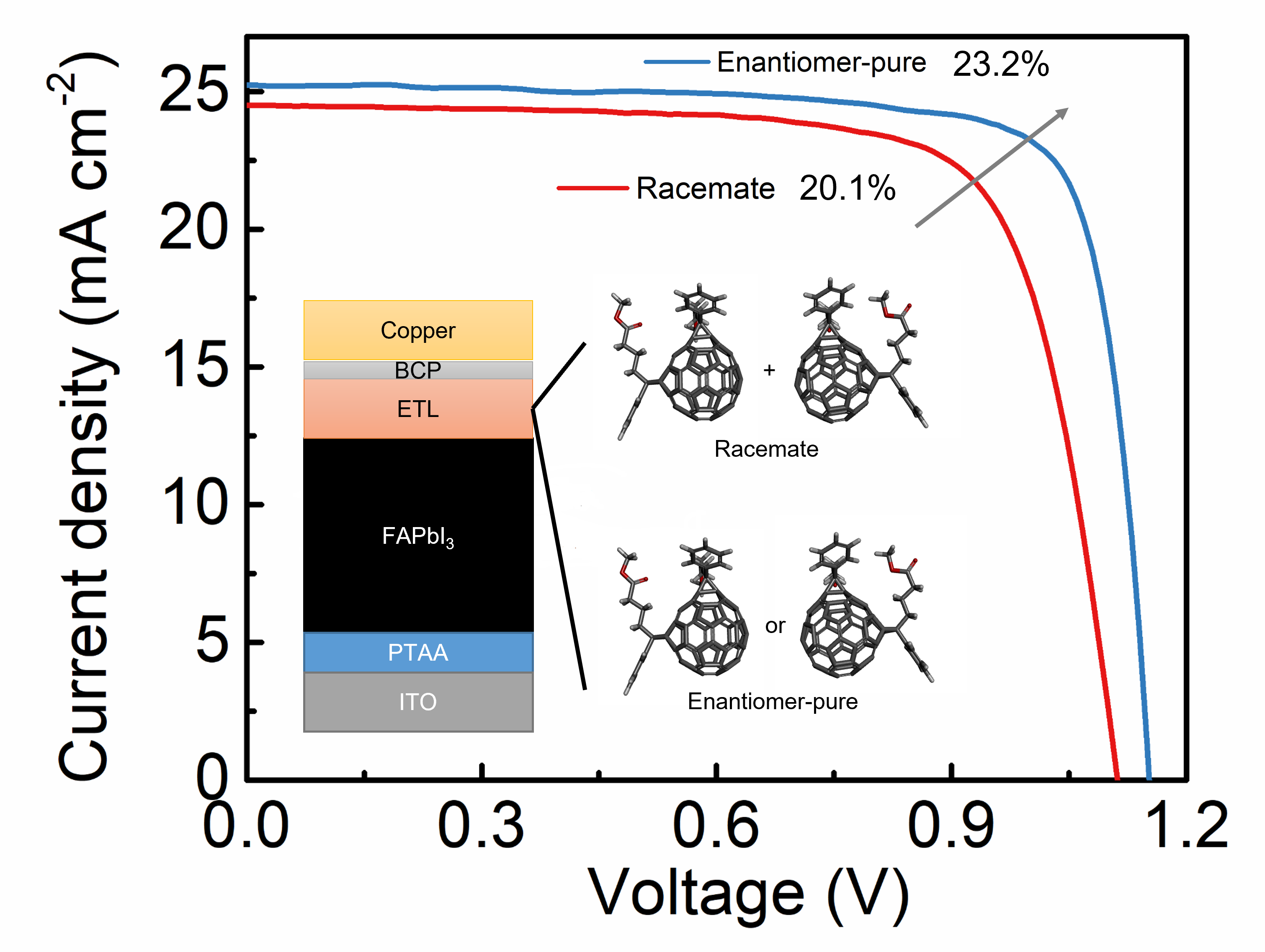 More recently, we have shown that chiral composition can play a large role in the performance of chiral fullerene electron-transport layers within the context of perovskite solar cells (PSCs). We prepared inverted PSCs using our chiral fullerene materials, comparing the performance of enantiomerically pure material to the corresponding racemate. The single enantiomer devices are found to have an improved performance, giving a power conversion efficiency (PCE) of 23.2%, compared to 20.1% PCE for the racemate. Such excellent performance for the single enantiomer devices is accompanied by enhanced operational stability. This study suggests that single isomer ETLs can provide important improvements in PSC performance and it positions chiral fullerenes as an exciting material class moving forward. Representative publications: Adv. Energy Mater. 2023, in press. DOI; ACS Nano 2017, 11, 8329. DOI.
More recently, we have shown that chiral composition can play a large role in the performance of chiral fullerene electron-transport layers within the context of perovskite solar cells (PSCs). We prepared inverted PSCs using our chiral fullerene materials, comparing the performance of enantiomerically pure material to the corresponding racemate. The single enantiomer devices are found to have an improved performance, giving a power conversion efficiency (PCE) of 23.2%, compared to 20.1% PCE for the racemate. Such excellent performance for the single enantiomer devices is accompanied by enhanced operational stability. This study suggests that single isomer ETLs can provide important improvements in PSC performance and it positions chiral fullerenes as an exciting material class moving forward. Representative publications: Adv. Energy Mater. 2023, in press. DOI; ACS Nano 2017, 11, 8329. DOI.
Controlling anisotropy
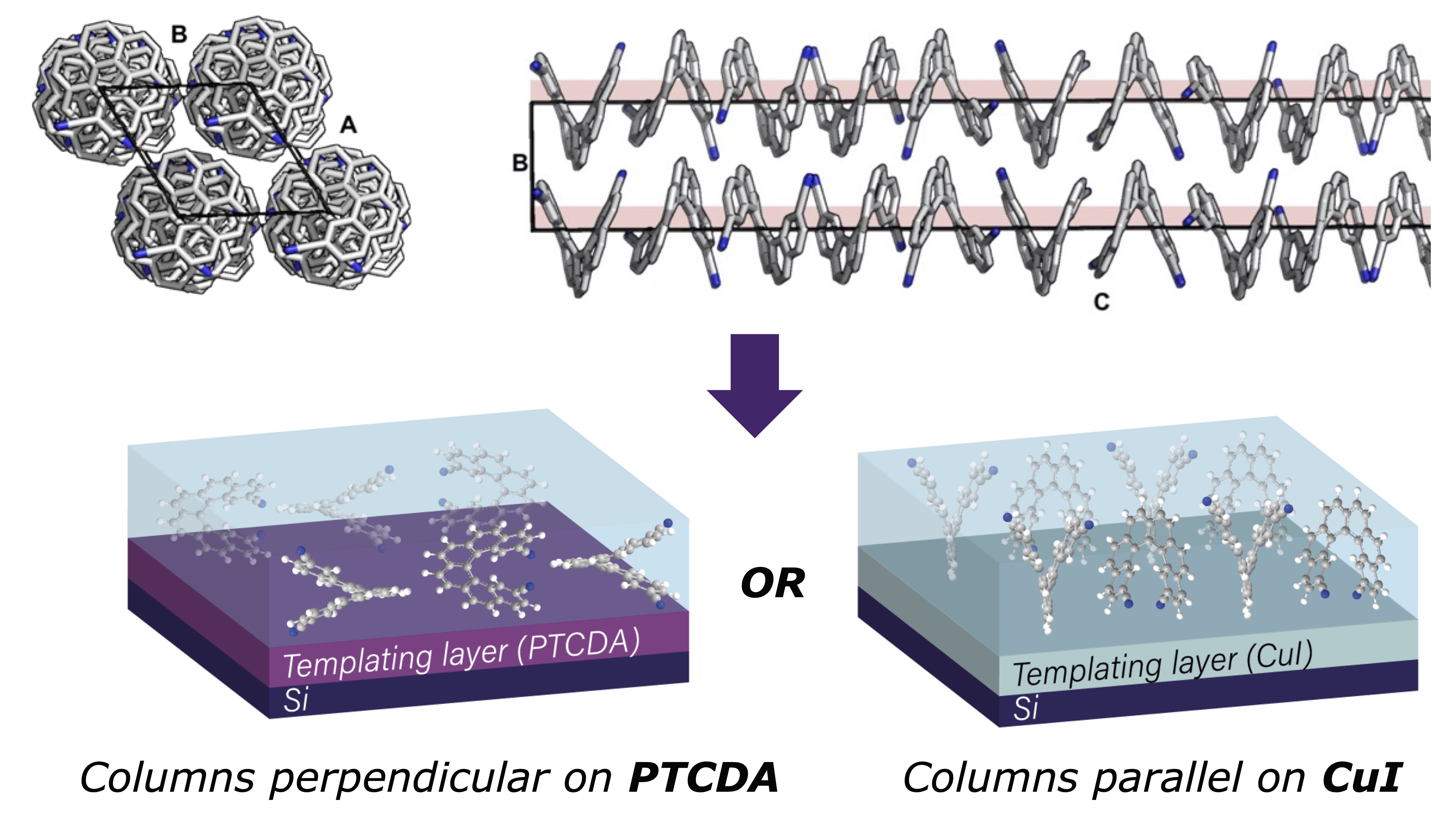
One challenge in the area of chiral materials is the intrinsically anisotropic (directional) nature of chiral-dependent properties, such as the absorption and emission of CP light or the transport of spin-polarised electrons. As a result, the orientation of chiral molecules relative to other interfaces, along with the directionality of the measurement, are critical to determine the functionality and efficiency of chiral materials and devices. We recently reported a strategy to control the orientation of a helicene by using organic and inorganic templating layers. Such templating layers can either force the helicene to adopt a face-on orientation and self-assemble into upright supramolecular columns oriented with their helical axis perpendicular to the substrate, or an edge-on orientation with parallel-lying supramolecular columns. Through such control, we show that low- and high-energy chiroptical responses can be independently ‘turned on’ or ‘turned off’. The templating methodologies described here provide a simple way to engineer orientational control and, by association, anisotropic functional properties of chiral molecular systems for a range of emerging technologies. Representative publication: Nature Chem. 2022, 14, 1383. DOI.