Critical illness
 Researchers from Imperial College have made substantial contributions to the field of paediatric severe life-threating illness in sub-Saharan Africa. They have highlighted the unique importance of emergency-care research as a highly targeted and cost-effective means of tackling childhood mortality in resource-limited sub-Saharan Africa, previously neglected as an area for specific funder or policy investment. Over the last 15 years, Professor Maitland’s group has undertaken pioneering clinical investigative research in resource-poor settings in the fields of severe malaria, sepsis, severe malnutrition and severe anaemia, by undertaking high-quality physiological studies and clinical trials, generating the essential evidence base for patient management and informing both national and international policy. To enable such translational research to occur, she has built up a network of African sites, investing in both training and local capacity development, bringing sophisticated technologies and applying them in real-life circumstances to study common and important diseases of Africa. Currently, her group have active collaborations across Africa, within Europe, South East Asia and Australia.
Researchers from Imperial College have made substantial contributions to the field of paediatric severe life-threating illness in sub-Saharan Africa. They have highlighted the unique importance of emergency-care research as a highly targeted and cost-effective means of tackling childhood mortality in resource-limited sub-Saharan Africa, previously neglected as an area for specific funder or policy investment. Over the last 15 years, Professor Maitland’s group has undertaken pioneering clinical investigative research in resource-poor settings in the fields of severe malaria, sepsis, severe malnutrition and severe anaemia, by undertaking high-quality physiological studies and clinical trials, generating the essential evidence base for patient management and informing both national and international policy. To enable such translational research to occur, she has built up a network of African sites, investing in both training and local capacity development, bringing sophisticated technologies and applying them in real-life circumstances to study common and important diseases of Africa. Currently, her group have active collaborations across Africa, within Europe, South East Asia and Australia.
Children's Oxygenation Administration Strategies Trial
Children's Oxygenation Administration Strategies Trial
 COAST: Children's Oxygenation Administration Strategies Trial is a randomised controlled trial designed to provide the evidence for the most clinically effective and cost-efficient targeted use of oxygen as a life-saving treatment with respect to the optimal oxygen saturation threshold for treatment and mode of delivery in African children with pneumonia complicated by hypoxia (SaO2< 92%).
COAST: Children's Oxygenation Administration Strategies Trial is a randomised controlled trial designed to provide the evidence for the most clinically effective and cost-efficient targeted use of oxygen as a life-saving treatment with respect to the optimal oxygen saturation threshold for treatment and mode of delivery in African children with pneumonia complicated by hypoxia (SaO2< 92%).
Need for a trial
While oxygen, as a potentially life-saving treatment, is advocated by the WHO, it has not been afforded a high enough priority for either country or global levels. Use of pulse oximeters are limited and sustainable supply of oxygen (either bottled or oxygen concentrators) is both expensive and logistically challenging. As a consequence, poorly targeted use results in few children, who may benefit from oxygen therapy, actually receive it. Systematic and policy reviews indicate the need for a formal evaluation of the hypoxia threshold at which oxygen should be targeted and of how oxygen is best administered. COAST was therefore designed on behalf of the International Severe Acute Respiratory Infection Consortium (ISARIC)to address these critical research gaps to provide a better evidence base for future guidelines, intending to improving the poor outcomes.
Trial Summary
Children will be enroled at admission to hospital over two years from 5 sites in 3 countries (Uganda, Kenya and Democratic Republic of Congo) and followed for 28 days. The trial has a practical design, to ensure this encompasses a spectrum of high-risk children, identified largely by clinical criteria, so that the results apply to health services in Africa, with limited access to health technologies.
Funders: COAST is funded (£2.7Million) by Joint Global Health Trial Scheme (Wellcome Trust MRC Dfid). Imperial College is the trial sponsor (formalised through a Heads of Terms).
Trial Partners:
Imperial College, London: Professor Kathryn Maitland is the Principal Investigator of COAST, and Professor Andy Bush (co-Investigator) will be responsible for overall trial coordination and scientific oversight of substudies. Kath Maitland is based full time in East Africa.
Africa: Prof S Kiguli, Dr Olupot Olupot, Dr Engoru, the three site PIs in Uganda, Dr Pat Njuguna Site PI KEMRI-Wellcome Trust Programme, Kilifi and Dr Marie Onyamboko Kishasa, DRC who are responsible for day-to-day management of the trial
ICNARC, UK Prof Kathy Rowan and David Harrison are responsible for test management and statistical support
John Fraser (Critical Care Group; Brisbane) and Fisher & Paykel, (industry leaders in high flow oxygen delivery), have agreed to donate the AIRVO devices for the trial. ISARIC, supported by Wellcome Trust, MRC and other funders, is a recently founded consortium of global researchers, including 70 established networks, to address critical research gaps and identify the most efficient treatments; its first strategic aim is to make a difference in severe acute respiratory infection (http://isaric.tghn.org).
Trial Summary
4,200 children aged between 28 days and 12 years with respiratory distress (putative pneumonia) and hypoxia (defined as SaO2 <92%) will be enroled at admission to hospital over two years from 5 sites in 3 countries (Uganda, Kenya and Democratic Republic of Congo) and followed for 28 days. The primary outcome is mortality at 48 hours.
Objectives
- To establish whether liberal oxygenation for SaO2 ≥80% will decrease mortality (at 48 hours and up to 28 days) compared with a strategy that includes permissive hypoxia (usual care); and
- To establish whether use of high flow oxygen delivery will decrease mortality (at 48 hours and up to 28 days) compared with low-flow oxygen delivery (usual care)
The trial has two strata:
Stratum 1: severe hypoxia, SaO2 <80%); and Stratum 2: hypoxia SaO2 ≥80% and <92%).
Children in Stratum 1 (2-arm, 1:1 ratio) will all receive oxygen and randomisation will allocate participants to one of two methods of oxygen delivery:
- high flow oxygen delivery
- low flow (usual practice) oxygen delivery
Children in Stratum 2 (3-arm, 2:1:1 ratio) will be allocated to:
- permissive hypoxia (no immediate oxygen): control
- high flow oxygen delivery
- low-flow oxygen delivery
Recruitment will start following all ethics and national approvals in January 2017 and complete in 2020. The trial has a practical design, to ensure this encompasses a spectrum of high-risk children, identified largely by clinical criteria, so that the results apply to health services in Africa, with limited access to health technologies.
FEAST trial
Fluid Expansion as a Supportive Therapy
The FEAST: Fluid Expansion as a Supportive Therapy trial enrolled 3,170 critically ill children and shock at six hospitals in Uganda, Kenya and Tanzania to evaluate the impact of a potentially life saving therapy fluid resuscitation (20-40mls/kg) or "boluses" of saline or albumin solutions, given rapidly over the first hour of admission to hospital. These were compared to children who did not receive boluses (control). This was the first time anywhere in the world that the treatment, known as Fluid Resuscitation, has been evaluated for safety and effectiveness in a large randomised clinical trial, despite the fact that it has been standard practice for the last two decades in much of the world, including the United States, Europe and Australasia. 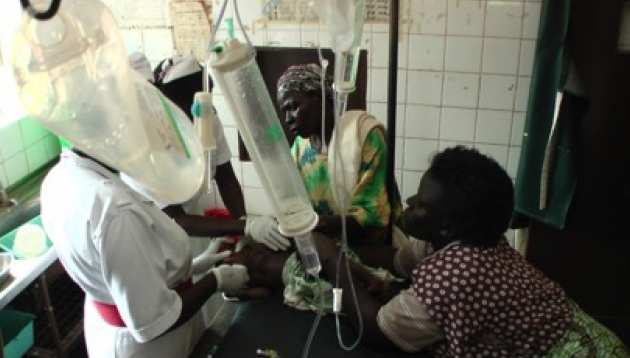
All the children in the trial had severe febrile illnesses, particularly malaria (in around 60% children) and septicaemia (a bacterial bloodstream infection). Combined, these are now the biggest causes of death in children under 5-years, claiming an estimated 2 million young lives every year. Of importance, children with shock caused by diarrhoea, severe malnutrition, burns and traumatic injuries -other major killers, were not included in the trial and results cannot therefore be extrapolated to children with these diseases.
The trial was stopped early by the committee overseeing the accumulating results of the trial (the data monitoring committee). The results of the FEAST trial, (Fluid Expansion As Supportive Therapy) was published in the New England Journal of Medicine in May 2011. It demonstrated that a long standing and key treatment used in wealthy countries to resuscitate critically ill children with shock, is harmful when given to African children with shock. In those receiving boluses 89.4% survived the first 48 hours in hospital, whereas children given fluids slowly (control group) did better; 92.7% of them survived. This was a statistically significant difference. This means that compared to no boluses (control group) boluses cause more than 3 children (3.3%) to die out of every one hundred treated.
https://www.youtube.com/watch?v=hK9VUkL-DqU
There has been considerable debate over the generalisablility of the results from FEAST. Numerous commentaries followed its publication in NEJM in 2011 and it was widely praised for demonstrating how rigorously clinical research can be performed in resource poor settings. In 2012 FEAST won the prestigious BMJ Research Paper of the Year award (links to BMJ?)). Extrapolating from the FEAST mortality results, the trial is likely to avert the deaths of thousands of children each year in Africa alone.
Policy briefs have also been commissioned and is available from Prof Maitland and our collaborators at the MRC CTU (website) packaged together with the results papers from FEAST, a systematic review of the evidence, and commentaries discussing the FEAST results and implications, into a FEAST Information Pack.
FEAST trial 10 key publications
- Maitland K, Akech SO, Russell EC: Mortality after Fluid Bolus in African Children with Sepsis: Reply. N Engl J Med 2011, 365:1348-1353, 10.1056/NEJMc1108712,
- Maitland K, Molyneux S, Boga M, Kiguli S, Lang T: Use of deferred consent for severely ill children in a multi-centre phase III trial. Trials 2011, 12:90, 10.1186/1745-6215-12-90, 3077324
- Ford N, Hargreaves S, Shanks L: Mortality after fluid bolus in children with shock due to sepsis or severe infection: a systematic review and meta-analysis. PloS one 2012, 7:e43953, 10.1371/journal.pone.0043953, 3431361
- Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, Opoka RO, Engoru C, Nyeko R, Mtove G, Reyburn H, Brent B, Nteziyaremye J, Mpoya A, Prevatt N, Dambisya CM, Semakula D, Ddungu A, Okuuny V, Wokulira R, Timbwa M, Otii B, Levin M, Crawley J, Babiker AG, Gibb DM: Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med 2013, 11:68, 10.1186/1741-7015-11-68, 3599745
- Molyneux S, Njue M, Boga M, Akello L, Olupot-Olupot P, Engoru C, Kiguli S, Maitland K: 'The words will pass with the blowing wind': staff and parent views of the deferred consent process, with prior assent, used in an emergency fluids trial in two african hospitals. PloS one 2013, 8:e54894, 10.1371/journal.pone.0054894, 3569446
- Todd J, Heyderman RS, Musoke P, Peto T: When enough is enough: how the decision was made to stop the FEAST trial: data and safety monitoring in an African trial of Fluid Expansion As Supportive Therapy (FEAST) for critically ill children. Trials 2013, 14:85, 10.1186/1745-6215-14-85, 3617035
- Kiguli S, Akech SO, Mtove G, Opoka RO, Engoru C, Olupot-Olupot P, Nyeko R, Evans J, Crawley J, Prevatt N, Reyburn H, Levin M, George EC, South A, Babiker AG, Gibb DM, Maitland K: WHO guidelines on fluid resuscitation in children: missing the FEAST data. BMJ 2014, 348:f7003, 10.1136/bmj.f7003,
- George EC, Walker AS, Kiguli S, Olupot-Olupot P, Opoka R, Engoru C, Akech SO, Nyeko R, Mtove G, Berkley JA, Mpoya A, Levin M, Crawley J, Gibb DM, Maitland K, Babiker A: Predicting mortality in sick African children: a clinical bedside risk score from the FEAST trial. BMC Med 2015, in press,
- Kiguli S, Maitland K, George EC, Olupot-Olupot P, Opoka RO, Engoru C, Akech SO, Nyeko R, Mtove G, Reyburn H, Levin M, Babiker AG, Gibb DM, Crawley J: Anaemia and blood transfusion in African children presenting to hospital with severe febrile illness. BMC Med 2015, 13:21, 10.1186/s12916-014-0246-7, 4313469
- Olupot-Olupot P, Engoru C, Uyoga S, Macharia A, Kiguli S, Opoka R, Akech S, Nyeko R, Mtove G, Nteziyaremye J, Chebet M, George EC, AG B, Gibb DM, Williams TN, Maitland K: High frequency of blackwater fever among children presenting to hospital with severe febrile illnesses in Eastern Uganda. Lancet Haematology 2015:under review.
We have produced a 40-minute documentary called FEAST: An Anatomy of a clinical trial. This film uses FEAST as an example to explain how a clinical trial works, and why we need to do randomised controlled trials. This movie is available on YouTube, the MRCCTU website and the Global Health Trials Network website, which has more than 100,000 users.
The TRansfusion and TReatment of severe Anaemia in African Children
The TRansfusion and TReatment of severe Anaemia in African Children
The TRansfusion and TReatment of severe Anaemia in African Children: a randomised controlled Trial
TRACT ISRCTN84086586 is a randomised controlled trial involving 3954 children aged 2 months to 12 years with severe anaemia (SA) (defined as a haemoglobin (Hb) <6g/dl). Children will be enroled at admission to hospital over 2 years from 2 countries (Malawi, Uganda) and followed for 6 months to make sure longer-term outcomes are captured. The trial has been designed to address the poor outcomes following SA in children in sub-Saharan Africa, which is associated with high rates of in-hospital mortality (9-10%), 6-month case fatality (12%) and relapse or re-hospitalisation (6%) indicating that the current recommendations and/or management strategies are not working in practice.
TRACT trial is designed to answer 3 simple questions:
- Which children should receive a transfusion? Current WHO guidelines, designed to avoid overuse of blood, recommend transfusions only in children with a Hb <4g/dl (or <6g/dl if accompanied by complications). These specific recommendations have not been evaluated in clinical trials and thus, practice varies across African countries. We don’t know if giving blood to all children with Hb <6g/dl would help.
- How much blood should be given in a transfusion? On current recommendations, a quarter of children receiving transfusions remain severely anaemic and up to one-third get two or more blood transfusions during a single hospital admission. We don’t know if giving larger initial volumes of blood would help – this could reducing risks from additional transfusion (which include bad blood matching or blood infections), and the amount of time health personnel spend getting blood ready.
- Would long-term support for children after hospital admission help? The major factors related to poor longer term outcome are multiple vitamin and mineral deficiencies and blood infections caused by bacteria - we don’t know if giving vitamin/mineral supplements or antibiotics to prevent infections would improve outcomes.
The TRACT trial will simultaneously look at three ways management of SA might be improved – with the aim of reducing early and late deaths, and anaemia recurrence or readmission to hospital. The trial has 3 simultaneous randomizations that compare:
- current conservative WHO recommendations for transfusion against a more liberal approach, in terms of who gets blood and how much blood they get
- additional multi-vitamin multi-mineral supplements compared with the standard folate/iron recommended by WHO and
- an antibiotic, cotrimoxazole, to prevent new bacterial infections for 3 months compared with no antibiotic.
The primary outcome is cumulative mortality to 4 weeks (transfusion comparisons) and to 6 months for the nutritional support/antibiotic prophylaxis comparison. Secondary outcomes include mortality at 48 hours, 4 weeks, 3 months and 6 months (cumulative) (where not the primary outcome); development of new profound anaemia (Hb<4g/dl) during acute admission or development of SA (Hb<6g/dl) post discharge; readmission to hospital; proportion achieving correction of anaemia (Hb>9g/dl); adverse events relating to transfusion.
The design has a pragmatic design with broad, largely clinical inclusion criteria. So when the results are published they will form a cheap and widely available ‘bundle’ of effective interventions, directed at both the immediate and downstream consequences of severe anaemia and which is likely to lead to substantial reductions in mortality in African children hospitalised with SA every year if widely implemented. The trial started enrolment in September 2014 and is on target for completion in December 2016.
The trial featured on BBC Health News.
TRACT Key References
- Mpoya A, Kiguli S, Olupot-Olupot P, Opoka RO, Engoru C, Mallewa M, Chimalizeni Y, Kennedy N, Kyeyune D, Wabwire B, M'Baya B, Bates I, Urban B, von Hensbroek MB, Heyderman R, Thomason MJ, Uyoga S, Williams TN, Gibb DM, George EC, Walker AS, Maitland K: Transfusion and Treatment of severe anaemia in African children (TRACT): a study protocol for a randomised controlled trial. Trials 2015, 16:593, 10.1186/s13063-015-1112-4, PMC4696199
- Kiguli S, Maitland K, George EC, Olupot-Olupot P, Opoka RO, Engoru C, Akech SO, Nyeko R, Mtove G, Reyburn H, Levin M, Babiker AG, Gibb DM, Crawley J: Anaemia and blood transfusion in African children presenting to hospital with severe febrile illness. BMC Med 2015, 13:21, 10.1186/s12916-014-0246-7, 4313469
- Olupot-Olupot P, Engoru C, Thompson J, Nteziyaremye J, Chebet M, Ssenyondo T, Dambisya CM, Okuuny V, Wokulira R, Amorut D, Ongodia P, Mpoya A, Williams TN, Uyoga S, Macharia A, Gibb DM, Walker AS, Maitland K: Phase II trial of standard versus increased transfusion volume in Ugandan children with acute severe anemia. BMC Med 2014, 12:67, 10.1186/1741-7015-12-67, 4101869
- Ala F, Allain JP, Bates I, Boukef K, Boulton F, Brandful J, Dax EM, El Ekiaby M, Farrugia A, Gorlin J, Hassall O, Lee H, Loua A, Maitland K, Mbanya D, Mukhtar Z, Murphy W, Opare-Sem O, Owusu-Ofori S, Reesink H, Roberts D, Torres O, Totoe G, Ullum H, Wendel S: External financial aid to blood transfusion services in sub-Saharan Africa: a need for reflection. PLoS medicine 2012, 9:e1001309, 10.1371/journal.pmed.1001309, 3439367
- Hassall O, Maitland K, Pole L, Mwarumba S, Denje D, Wambua K, Lowe B, Parry C, Mandaliya K, Bates I: Bacterial contamination of paediatric whole blood transfusions in a Kenyan hospital. Transfusion 2009, 49:2594-2598, 10.1111/j.1537-2995.2009.02344.x, 2939982
- Akech SO, Hassall O, Pamba A, Idro R, Williams TN, Newton CR, Maitland K: Survival and haematological recovery of children with severe malaria transfused in accordance to WHO guidelines in Kilifi, Kenya. Malaria journal 2008, 7:256, 10.1186/1475-2875-7-256, 2615447
International Severe Acute Respiratory and Emerging Infection Consortium
International Severe Acute Respiratory and Emerging Infection Consortium
ISARIC - International Severe Acute Respiratory and Emerging Infection Consortium - is a global initiative aiming to ensure that clinical researchers have the open access protocols and data-sharing processes needed to facilitate a rapid response to emerging diseases that may turn into epidemics or pandemics.
Gathering over 70 networks and individuals involved the consortium is trying to understand the causes of severe acute respiratory diseases, discover how illnesses develop and progress in patients, and identify the most efficient treatments and the best way to prevent further transmission and includes research related to the outbreaks of diseases such as bird flu (H5N1), swine flu (H1N1) and SARS.
The overreaching ambition is to change the way in which research is carried out during and between epidemics, ISARIC aims to address the social and ethical issues related to this paradigm change. Following its launch in December 2011, ISARIC has been established with an online presence as well as a physical presence in its Secretariat, based at the Centre of Tropical Medicine, Oxford as of mid-May 2012. In its first quarter, more than seventy networks and consortia of over 30 countries across six continents expressed an interest in the work of ISARIC.
ISARIC provides a collaborative platform through which global, patient‐oriented clinical studies can be developed, executed and shared. ISARIC activities promote the standardisation and harmonisation of definitions, endpoints and data collection methods so that international results can be combined and compared. Research protocols and data tools addressing the most important questions between and during epidemics and rapidly emerging public health threats have been designed to generate new knowledge, maximise the availability of clinical information, and thereby save lives.
These tools are available for anyone to download.
From Imperial College: Professor Peter Openshaw (Working Group 3; Genomics, Pathogenesis and Pharmacology) and Professor Kath Maitland (African Chair) are members of the ISARIC consortium.




