Imperial Prostate
Contact
Clinical Director
Prof Hashim Ahmed
Email: hashim.ahmed@imperial.ac.uk
Twitter: @LondonProstate1
Research Enquiries
Email: research@imperialprostate.org
What we do
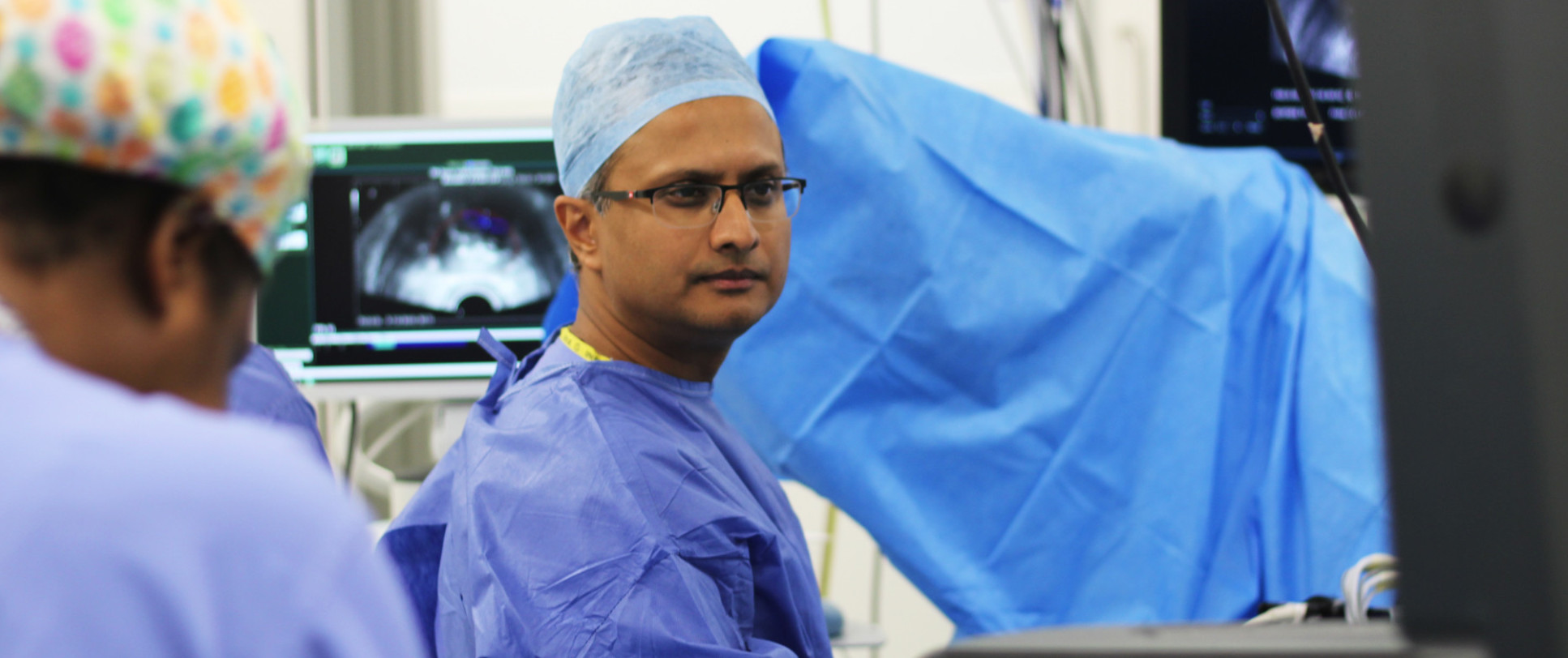
Imperial Prostate is a world-leading collective of expert urologists, medical specialists, and experienced researchers who are working towards improving the lives of men who develop or are being investigated for prostate cancer and other prostate diseases.
Our mission is to improve the speed, accuracy and safety of prostate disease diagnosis, and to evaluate new treatments which have fewer side effects than standard techniques without losing treatment efficacy. We are dedicated to helping ensure that the best model of prostate disease care is available to all men throughout the UK as well as worldwide.
Imperial Prostate is led by a core clinical team – Professor Hashim U. Ahmed, Mr Mathias Winkler, Mr Manit Arya and Mr Taimur Shah, and is based at Charing Cross Hospital, London. We are partnered with scientists at Imperial College London, who help us develop our research based on the latest scientific discoveries, techniques and technology. We also collaborate closely with the Imperial College Trials Unit (ICTU) to design, conduct and disseminate our clinical trials.
Follow Imperial Prostate on Twitter.
Research & Activity
- Evaluating New Technology
The potential of speedy, accurate and safe prostate diagnostics has been transformed by the development of multi-parametric MRI, the use of which has been pioneered by Professor Ahmed. The benefits of an image-based approach to prostate disease have now been proven by this work – the research group now works to push the limits of this technology even further, to further improve diagnosis of prostate disease while reducing the number of surgical operations and hospital visits for patients.
New technologies are constantly being developed for treating prostate cancer and other prostate diseases, and through partner NHS institutions we trial and evaluate these devices and technologies, such as High Intensity Focused Ultrasound (HIFU) treatment for prostate cancer, or injected water vapour (Rezūm) treatment for benign prostatic enlargement. This work is essential in order to provide evidence that they are safe and effective, and the data collected helps to determine which treatment is best in which circumstance, helping treatment be tailored to the individual patient.
- Education for Urologists
While medical research is essential for driving change for patients, so also is educating practitioners on these changes. Our team is committed to organising and producing educational events which allow for information on best practice to disseminate across the UK and worldwide, as well as provide a space for researchers and developers of cutting edge techniques to collaborate and discuss the current prostate research paradigm. Learn more about our annual Imperial Prostate Masterclass.
- Transforming Standard of Care
Another determining factor in delivering theoretical developments into real advances for patients is reliant on local hospitals having the material means and the knowledge from other successful hospitals to enact change in their unit.
We are running trials on new patient pathways and other organisational structures for hospital administrations to serve as a model of all-UK and worldwide hospitals, such as the RAPID clinic, so that every man having their prostate’s health checked can have the highest quality investigation in the minimum number of hospital trips and time spent waiting.
Summary of current research
IP1-PROSTAGRAM
PROSTAGRAM – The PROSTAGRAM Trial aimed to find an imaging technique, like mammograms for breast cancer, that could be offered as a screening test for prostate cancer. For the first time, mortality rates from prostate cancer have surpassed breast cancer which benefits from a screening programme offered to all women.
At present, there is no nationally approved population-based screening programme for prostate cancer. The most common test, prostate-specific antigen (PSA), is too unreliable for screening and it is not recommended for routine use in national screening guidelines. Yet the mortality rates for prostate cancer remain high, with 1 in 24 men dying from the disease.
Recent advances in prostate imaging have led to the development of new techniques which could be offered as screening tests. These new imaging tests are non-invasive, efficient and reliable. The tests which will be included in the PROSTAGRAM trial are:
- A ‘fast’ bi-parametric MRI. Less than 15 mins scanning time
- Ultrasound shearwave elastography (SWE). This measures the stiffness of the prostate and is cheaper than an MRI scan
- Standard of care PSA test
The trial addressed an unmet need for research into alternative screening tests to PSA. It was the first trial offering men a screening test with MRI and US, and a first step towards developing a national screening programme for prostate cancer.
IP2-ATLANTA
ATLANTA - The ATLANTA Trial aims to investigate the role of treating the local prostate tumour and metastasis in men with newly diagnosed metastatic prostate cancer. Men with advanced prostate cancer that has spread well beyond the gland itself (metastatic) are currently treated with drug therapy alone, such as chemotherapy. However, there is growing evidence that some men may benefit from having their local prostate tumour treated, even though it has already spread. In addition, some men may benefit from having selected distant cancer deposits treated.
ATLANTA will randomise men to two additional local treatment options Radical treatment (Surgery or Radiotherapy) OR Minimally Invasive Ablative Therapy (Prostate Cryotherapy or HIFU). In addition, some men in these treatment arms will receive specialised radiotherapy to cancer deposits, known as Stereotactic Ablative Radiotherapy (SABR). This is sometimes referred to by brand names such as Cyberknife© radiotherapy. All trial treatments will be in addition to the routine NHS standard of care treatment.
Men in the treatment arms will be compared to men who are undergoing routine NHS standard of care in the control arm. The trial will recruit for two years (April 2019 onwards), with a follow-up period of two years. We will be primarily looking at whether these additional treatments delay the progression of the disease and secondly, whether overall survival is improved.
ATLANTA is entirely charity-funded by the Wellcome Trust. The trial is available in 30 trial centres across the NHS in England & Wales. No man will be more than 30 minutes from an ATLANTA trial centre.
IP3-PROSPECT
PROSPECT – Traditionally, level one evidence is obtained from two-arm, or 3-arm, randomised controlled trials (RCT) of a new treatment(s) and compare it to an established treatment in which neither the patient nor the doctor knows who is taking what, until the end of the study (‘double-blind’). Unfortunately, in trials involving surgery or complex interventions, a variety of factors can lead to the failure to accrue sufficient participants in many traditional RCT designs. This has been particularly highlighted in localized prostate cancer RCTs, where 12 RCTs evaluating different interventions in localised prostate have failed to recruit, many in the last 5 years.
IP3-PROSPECT will explore a trial design called the cohort-multiple RCT (cmRCT). This design has been used in a number of disease areas, both benign and cancer. We have chosen prostate conditions since they are extremely common and if malignancy occurs the majority of men with the disease are regarded as living with a chronic condition due to its long natural history and in which innovative approaches, interventions, treatments or changes in management might have a significant patient benefit and impact on the NHS.
We want to test the acceptability and feasibility of the cmRCT in the prostate pathway. As this is the first time that we are trying out this method we need to first pilot it. In the first part of the study, we want to evaluate the following. What is the accrual rate? What do patients and their healthcare professionals think of the cmRCT design? Is the data we collect robust? What are the resource requirements of such a study? We will then test a number of novel interventions or changes in the pathway and compared them to standard care in the cohort that we recruit.
IP4-CHRONOS
CHRONOS – Focal ablative therapy for localised clinically significant prostate cancer is an emerging treatment modality due to the improved post-procedural morbidity rates, and promising short-medium term cancer control outcomes. Though available on the NHS in limited centres, it is not readily available to all patients. The reason for this is the lack of high-quality trial-based evidence comparing focal therapies against radical whole gland treatments. Attempts of randomised control trials have been unsuccessful, primarily due to lack of acceptance of randomisation treatment allocation, and lack of physician equipoise. The unique structure of CHRONOS using side by side RCTs allow patient preference and physician equipoise to be considered will ensure maximal recruitment and retention of eligible patients.
CHRONOS A will directly compare radical therapies to focal therapies, the aim of which is to directly assess both cancer control and functional outcomes of radical therapies to focal therapy. CHRONOS B will compare focal therapy, to focal therapy with neoadjuvant treatment, the aim of which is to improve the re-do rates observed in previous studies.
IP5-MATTER
MATTER - Using a prospective, multicentre discrete choice experiment, we aim to determine the attributes associated with treatment that are most important to men with Metastatic Hormone-Sensitive Prostate Cancer. Furthermore, we plan to determine men’s preferences for, and trade-offs between, the attributes (survival and side effects) of different treatment options including systemic therapy, local cytoreductive approaches (external beam radiotherapy, cytoreductive radical prostatectomy or minimally invasive ablative therapy) and metastases-directed therapies (metastasectomy or stereotactic ablative body radiotherapy).
Understanding men’s preferences for treatment options in this disease state is crucial for patients, clinicians, carers and future healthcare service providers.
IP6-CHAIROS
CHAIROS – In the UK, about 80-100,000 men every year undergo prostate biopsy to diagnose prostate cancer. This equates to approximately 4 million histology slides; this is estimated to increase to 160,000-200,000 men and up to 6 million slides by 2030 due to rising numbers of men being tested for prostate cancer.
Galen Prostate AI is a CE-marked deep learning AI-algorithm for prostate needle biopsies that can identify cell types, tissue structures and morphological features for cancer diagnosis. The technology is based on multi-layered convolutional neural networks (CNNs) designed for image classification in which whole-slide imaging is analysed for the detection of tissue areas and then benign versus cancer versus other pathology classification. Compared to almost all competitors, Galen Prostate AI has been tested in ~10 times more tissue samples. Further, Galen Prostate AI is the only algorithm that extends beyond cancer detection/grading to other clinically relevant features (e.g., perineural invasion, high-grade prostatic intraepithelial neoplasia [PIN], inflammation). To our knowledge, it is the only AI-algorithm in routine clinical deployment – demonstrating technical feasibility and with proven clinical utility.
The proposed study will perform validation in the NHS, for the first time. It is important to stress that this type of algorithm has never been tested on a UK-based population, and in particular, a population that includes a cohort of MRI targeted biopsies, which is now the new diagnostic strategy as it detects clinically relevant prostate cancer in higher percentages than the routine systematic biopsy.
IP7-PACIFIC
PACIFIC – The PACIFIC trial aims to evaluate the role of biparametric MRI and image-fusion targeted biopsies for the detection of prostate cancer. PACIFIC will evaluate the NHS healthcare burdens of bpMRI compared to mpMRI, and image-fusion technology compared to visual-registration targeting, in terms of adverse events, proportion of patients biopsied and proportion of patients diagnosed with clinically significant and insignificant cancers that do not require treatment. It also aims to determine the impact of bpMRI and image-fusion targeting on detecting clinically significant cancer using other commonly used histological definitions of clinical significance.
bpMRI versus mpMRI - In those patients suspected to have prostate cancer, to determine whether bi-parametric MRI (bpMRI), compared to multi-parametric MRI (mpMRI), is able to accurately rule-out and detect clinically significant prostate cancer (any Gleason score >/=7 [ISUP Grade Group >/=2]), without increasing the number of patients biopsied or diagnosed with clinically insignificant prostate cancer.
Visual-registration targeting versus image-fusion targeting - In those patients recommended to have a prostate biopsy due to a suspicious MRI (bpMRI or mpMRI), to determine if new technology using MRI to ultrasound image-fusion to carry out targeted prostate biopsies is better at detecting clinically significant prostate cancer (any Gleason score >/=7 (any Gleason score >/=7 [ISUP Grade Group >/=2]), compared to biopsies carried out using visual-registration targeting.
HEAT and ICE Registry
Focal therapy is permissible per NICE IPG 423 and 424 guidance outside of trial settings provided outcomes are documented within a registry (1, 2). Within the UK, centres providing focal therapy for prostate cancer record oncological and safety outcomes within anonymised multi-centre registries called HIFU Evaluation and Assessment of Treatment (HEAT) and ICE (cryotherapy). These registries rely on charity funding, not corporations or industry partners therefore have no bias towards outcomes recorded.
Currently, 10 HIFU and 8 cryotherapy sites have inputted thousands of focal ablative cases into the HEAT and ICE registries respectively, allowing collaborative research evaluating treatment outcomes- most recently allowing the publication of a comparison between focal therapy against radical prostatectomy for localised prostate cancer. Such collaborative evaluations are important for assessing local outcomes to the UK average, as well as determining areas of improved treatment delivery and enabling providers to better counsel patients pre-operatively.
Our team
Professor Hashim Ahmed
/prod01/channel_2/media/migration/faculty-of-medicine/hash-armed-2-tojpeg-1502060122560-x1_1612975850338_x4.jpg)
Professor Hashim Ahmed
Chair in Urology (Clinical)
Mathias Winkler
/prod01/channel_2/media/migration/faculty-of-medicine/mathias-winkler_1612976770242_x4.jpg)
Mathias Winkler
Consultant urologist
Mr Manit Arya
/prod01/channel_2/media/migration/faculty-of-medicine/mr-manit-arya-1_1642087353656_x4.jpg)
Mr Manit Arya
Consultant Urologist
Mr Taimur Shah
/prod01/channel_2/media/migration/faculty-of-medicine/1634199205626_1642087491100_x4.jpg)
Mr Taimur Shah
Consultant Urologist
Martin Connor
/prod01/channel_2/media/images/people-list-300X400/fwxginzxeaaolpc-2_1658583640448_x1.jpg)
Martin Connor
Clinical Lecturer
Alexander Light
/prod01/channel_2/media/images/people-list-300X400/4fd32d28-4d6e-413e-941c-a1ff062e8862_1681243114694_x1.jpg)
Alexander Light
Honorary Academic Clinical Fellow
Nikhil Mayor
/prod01/channel_2/media/migration/faculty-of-medicine/holding-png-tojpeg-1564655919889-x2_1612975946329_x4.jpg)
Nikhil Mayor
Academic Clinical Fellow
Increase Akinyemi
/prod01/channel_2/media/images/people-list-300X400/Holding-PNG--tojpeg_1564655919889_x2.jpg)
Increase Akinyemi
Clinical Trial Manager
Johanna Sukumar
/prod01/channel_2/media/images/people-list-300X400/Holding-PNG--tojpeg_1564655919889_x2.jpg)
Johanna Sukumar
Clinical Trial Manager
Muna Osman
/prod01/channel_2/media/migration/faculty-of-medicine/holding-png-tojpeg-1564655919889-x2_1612976079591_x4.jpg)
Muna Osman
Clinical Research Facilitator
Puja Jadav
/prod01/channel_2/media/images/non-standard-dimensions/puja-headshot-mar24_1710169158123_x1.jpg)
Puja Jadav
Clinical Trial Manager
Francesca Rawlins
/prod01/channel_2/media/migration/faculty-of-medicine/holding-png-tojpeg-1564655919889-x2_1612976430072_x4.jpg)
Francesca Rawlins
Senior Clinical Research Practitioner
Emma Cullen
/prod01/channel_2/media/migration/faculty-of-medicine/holding-png-tojpeg-1564655919889-x2_1642090093961_x4.jpg)
Emma Cullen
Research Team Manager
Ross Dalton
/prod01/channel_2/media/images/people-list-300X400/silhouette--t_1430232718181_x2--t_1458213756113_x2--t_1489747983917_x2--tojpeg_1520949330649_x1.jpg)
Ross Dalton
Clinical Trials Coordinator


