

Solar panels could be improved by arranging their molecules in a certain pattern, according to new research led by Imperial and UCL.
Renewable solar power can be harnessed using solar cells, which are made of materials called semiconductors. In a solar cell, electrons are generated when photons, or packets of light, are absorbed by semiconductors and released as high energy electrons.
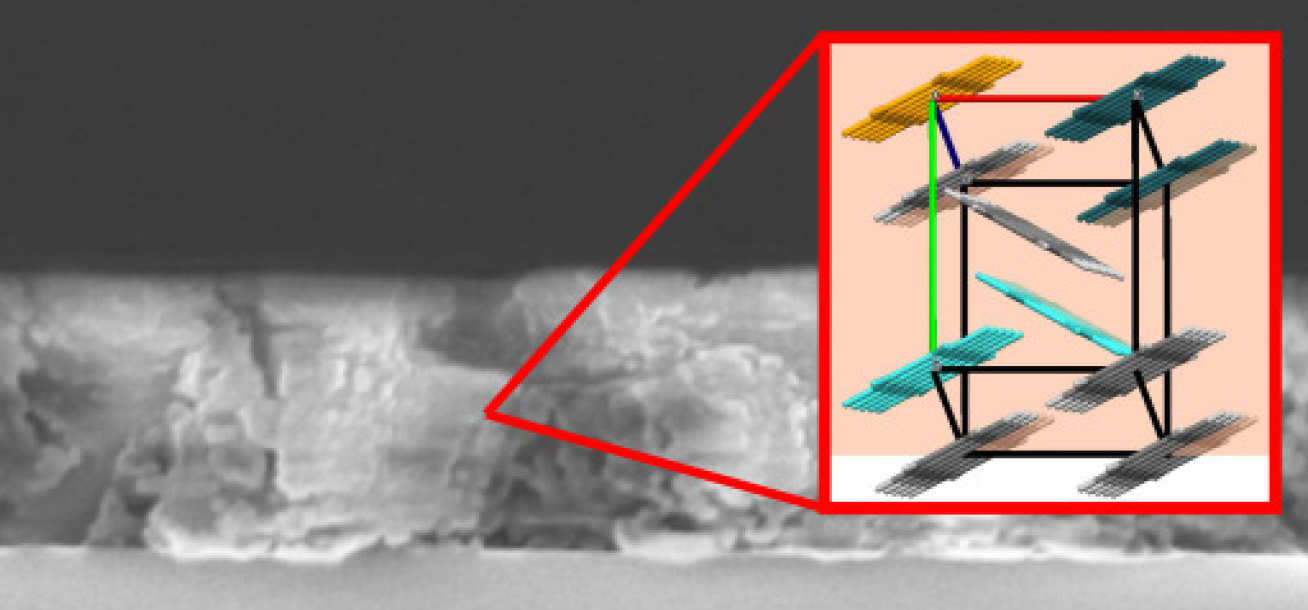
The way pentacene molecules are arranged in a solar cell can increase the energy output. This could make it much cheaper and more efficient to harvest solar energy in the long run. Daphné Lubert-Perquel Department of Materials
However, in some materials, one photon can generate two excited electrons – a highly unusual phenomenon known as singlet fission. In this case not all the electrons are able to escape the semiconductor and to be used as electricity.
Now, researchers based at Imperial College London and UCL’s joint London Centre for Nanotechnology have identified the best pattern for how molecules of the widely used semiconductor, pentacene, should be arranged to release the most electrons possible during singlet fission. The findings are published in Nature Communications.
Lead author of the paper Daphné Lubert-Perquel, from Imperial’s Department of Materials, said: “We found that the way pentacene molecules are arranged in a solar cell’s semiconductor can increase the energy output. This could make it much cheaper and more efficient to harvest solar energy in the long run.”
Power of pentacene
The pentacene layers used in solar cells have ‘herringbone’ structures, meaning neighbouring molecules of pentacene can be either parallel to each other or tilted at angles. Pentacene molecules are well known for their ability to undergo ‘singlet fission’ and therefore could generate double the electrons than photons absorbed.
The team, led by Professor Sandrine Heutz from Imperial’s Department of Materials, and UCL’s Professor Chris Kay, found that the parallel formation of pentacene molecules unleash more electrons than when the molecules are tilted towards one another.
When molecules are arranged in parallel, the excited electrons ‘escape’ more easily with the potential to form electrical currents.
This is the first time researchers have shown that the electron escape depends on pentacene’s molecular arrangement, and is also the first time scientists have conducted such experiments at room temperature.
Co-author Dr Enrico Salvadori, who carried out the work at UCL and is now at Queen Mary University of London, said: “Using a unique method to determine the pentacene orientation in our samples, we identified the parallel geometry and found it allows electrons to be released more easily. Conversely, the molecules in the herringbone or tilted geometry form traps for the electrons.
“This is the first time this work on pentacene was carried out at room temperature, allowing our findings to be applied in the real world, like in solar panels.”
Solar shapes
Sunlight is increasingly used as a source of renewable energy. Lubert-Perquel said: “By combining materials science and advanced spectroscopies we have generated key knowledge that will be crucial in developing new technologies for the solar industry. This is important to keep improving upon technology that provides us with renewable energy. ”
Next, the researchers will build on the work to control these molecular orientations and create a system with only the desired geometry. They hope to produce the best possible formation in multiple layers within a solar cell.
The work was funded by the UK Engineering and Physical Sciences Research Council (EPSRC). The work was done in a collaboration with researchers at Oxford University and Kobe University, Japan.
“Identifying triplet pathways in dilute pentacene” by Daphné Lubert-Perquel, Enrico Salvadori, Matthew Dyson, Paul N. Stavrinou, Riccardo Montis, Hiroki Nagashima, Yasuhiro Kobori, Sandrine Heutz, & Christopher W.M. Kay, published 11 October 2018 in Nature Communications.
Image credits:
Main image: Shutterstock/Sergey Molchenko
Article image: London Centre for Nanotechnology/Daphné Lubert-Perquel
Supporters

Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter

Caroline Brogan
Communications Division

Contact details
Tel: +44 (0)20 7594 3415
Email: caroline.brogan@imperial.ac.uk
Show all stories by this author




Leave a comment
Your comment may be published, displaying your name as you provide it, unless you request otherwise. Your contact details will never be published.