A bioengineering approach to find new treatment for bleeding and wound healing
A collaboration has led to a breakthrough in developing an engineered protein fragment that can help promote new blood vessels during wound healing.
Excessive bleeding continues to be a major complication of trauma, surgery or disease, often still leading to death. Stopping bleeding is also the important first step in the healing of wounds. A collaboration between Professor Randi of the National Heart and Lung Institute, and Professor Hubbell, of the University of Chicago, has led to a breakthrough in developing an engineered protein fragment that can help promote new blood vessels during wound healing. There are some diseases where increased, often spontaneous, bleeding is caused by genetic mutations (e.g. haemophilia). Professor Randi and her team have been studying a similar disease called Von Willebrand disease (VWD); this disease is more common than haemophilia and occurs in both men and women. It is caused by a lack, or disfunction, of Von Willebrand Factor (VWF), a protein found in your blood made by the cells lining blood vessels (endothelial cells). Studying this disease, the scientists have found a new way to promote the healing of wounds, opening the possibility of a simpler, cheaper and more effective treatment not only for patients with VWD but also for the wider population.
"This study is an example of how fundamental research can lead to unexpected discoveries which could improve people’s lives"
When skin is cut, scraped, or punctured, it starts to bleed. Bleeding also occurs during surgery. Small cells in blood called platelets are the first line of defence, swarming to the area to form a plug to stop the bleeding. The plug is at first temporary. An established plug then forms thanks to the ‘coagulation cascade’. The platelets need VWF to stick and form that first plug to stop the bleeding. People with reduced or malfunctioning VWF have a reduced ability to stem the flow of blood. This disease has been known about and treated effectively for a long time, mainly with injections of VWF. However, there are some patients for whom this is not an effective treatment. They have a particular type of bleeding from the gastro-intestinal tract which is difficult to stop with the injections of VWF. This version of the disease is not common but causes sufferers to have very severe bleeding and to require blood transfusions, leading to a poor quality of life and a risk of blood born infections. Professor Randi, in collaboration with Professor Mike Laffan (who runs the haemophilia clinic at Hammersmith Hospital), undertook an MRC funded study that discovered that one possible reason for this problem is that in healthy people VWF helps blood vessels to form and function properly. The ability to form and repair blood vessels (angiogenesis) is necessary for life, for example in the development and growth of the embryo, during menstruation and in wound healing, from a simple cut to surgery.
Their discovery showed that if you don’t have VWF, the blood vessels in the body are fragile and malformed, therefore prone to rupture. Resulting in you having two problems: platelets not sticking together to help form the plug that stops bleeding, and fragile blood vessels. The challenge has been to better understand how VWF works in blood vessels, so that new treatments for these patients can be found.
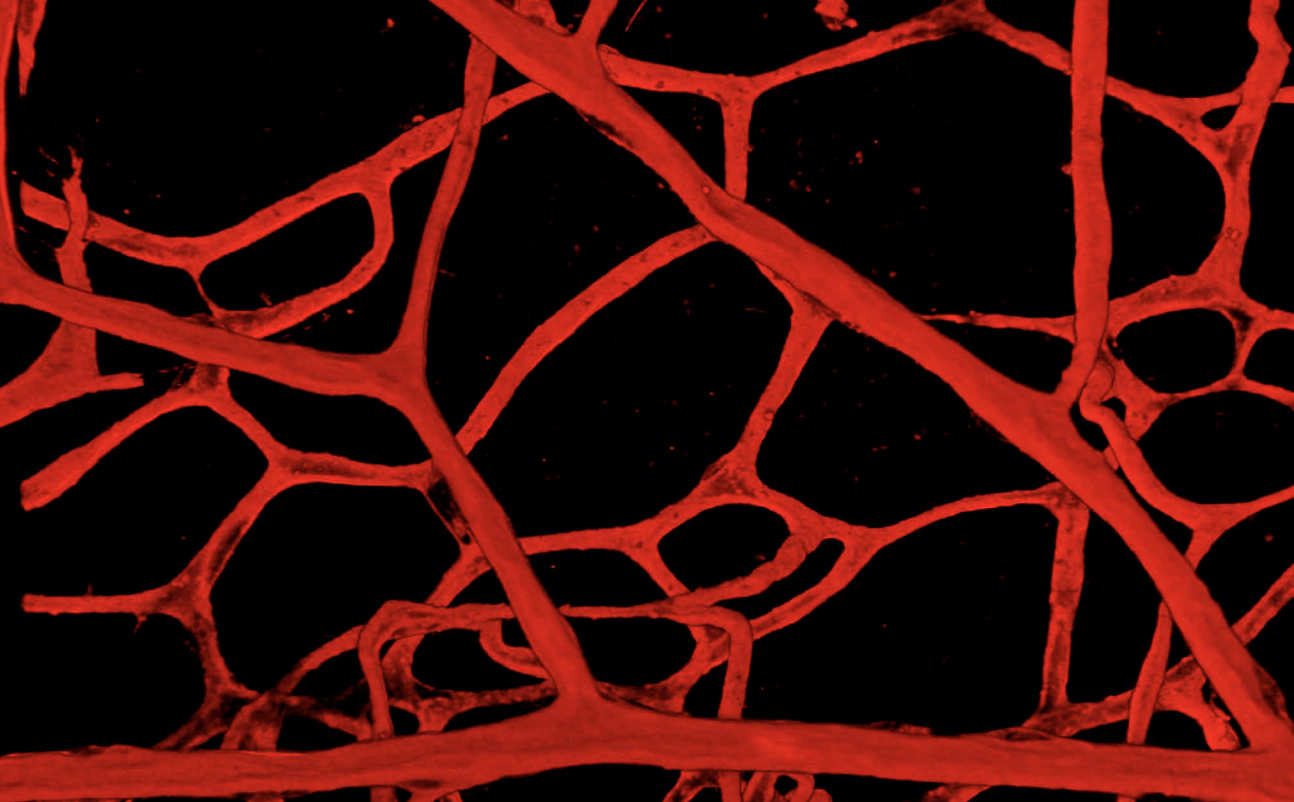 A few years after this discovery, Professor Randi heard Professor Jeffrey Hubbell speak at a conference about his work on molecules similar to VWF. Professor Hubbell is a bioengineer based in Chicago, USA, interested in engineering molecules to promote wound healing and tissue regeneration. They had been engineering protein fragments which could bind to the factors that promote blood vessels formation and angiogenesis. Professor Randi proposed that they could do the same thing with fragments of VWF, and so they set up a collaboration between her team at Imperial College London and Professor Hubbell's team in Chicago.
A few years after this discovery, Professor Randi heard Professor Jeffrey Hubbell speak at a conference about his work on molecules similar to VWF. Professor Hubbell is a bioengineer based in Chicago, USA, interested in engineering molecules to promote wound healing and tissue regeneration. They had been engineering protein fragments which could bind to the factors that promote blood vessels formation and angiogenesis. Professor Randi proposed that they could do the same thing with fragments of VWF, and so they set up a collaboration between her team at Imperial College London and Professor Hubbell's team in Chicago.
Their work, funded in the UK by the BHF, found a specific short region of VWF that can bind to the factors which promote the formation of blood vessels (called “growth factors”) and therefore wound healing. This was an important breakthrough as VWF is a very large protein which has many component sections, some with as yet unknown functions. The team had some clues that pointed to a specific region: this is a region of the protein which mediates its main functions, the same region that Professor Randi worked on as a PhD student in the early 1990s, in Professor Evan Sadler’s lab, at Washington University, St Louis, USA. Dr Jun Ishihara, a scientist in the Chicago team, produced a synthetic version of this short region of VWF and showed that it was capable of promoting wound healing, by forming a reservoir for growth factor within a wound and therefore facilitating the formation of blood vessels and the regeneration of the tissue. The ability to achieve this wound healing effect for treatment is a potent translational tool, which opens doors to potential future treatments.
Another exciting aspect of this discovery is that the fragment of VWF that could promote wound healing in Von Willebrand Disease patients, could also promote wound healing in the general population. Wound healing is a big problem, for instance in surgery and for diabetics. Therefore, this work could have a wide application in the general population. Further research is required to verify whether this approach works in humans, and how it can be used, but the possibilities are exciting. Could the engineered fragment help the intestinal bleeding of Von Willebrand Disease patients, will it be useful when they undergo minor surgery? Could it be widely applied in post-traumatic surgery to speed patient recovery and free up hospital beds? The researchers want to investigate whether this peptide could be engineered into a patch and thus make it much easier to treat patients, reducing risk and cost. This study is an example of how fundamental research can lead to unexpected discoveries which could improve people’s lives.
Read the full article 'The heparin binding domain of von Willebrand factor binds to growth factors and promotes angiogenesis in wound healing' in the journal Blood.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Ms Helen Johnson
Communications Division