Background
The discoidin domain receptors, DDR1 and DDR2, are receptor tyrosine kinases that function as collagen receptors. Both DDRs play important roles in developmental processes and a wide range of human diseases, for which only poor treatment options exist. There is a keen interest in anti-DDR drug discovery. However, significant gaps exist in our understanding of the basic mechanism of DDR kinase activation.
Research in the Leitinger lab

Our research has significantly contributed to a better understanding of the activation mechanism of the DDRs. We identified the collagen-binding site in the DDRs (Leitinger, 2003) and established their collagen-binding specificity (Leitinger et al, 2004; Leitinger and Kwan, 2006). We were the first to observe that the DDRs exist as dimers in the absence of ligand binding (Noordeen et al, 2006). This is in stark contrast to typical receptor tyrosine kinases that are thought to exist as monomers that dimerise following ligand binding. The fact that the DDRs are pre-dimerised and exist as constitutive dimers on the cell surface (Xu et al, 2014) established that DDR activation does not conform to the canonical receptor tyrosine kinase activation mechanism.
In collaboration with Richard Farndale, University of Cambridge, we identified specific DDR binding sites in collagens (Konitsiotis et al, 2008; Xu et al, 2011) and discovered that the DDRs promote integrin-mediated cell adhesion to collagen by enhancing integrin activation (Xu et al, 2012). In collaboration with Bassam Ali, United Arab Emirates University, UAE, we described the cellular and biochemical mechanisms underlying a severe human skeletal growth defect caused by missense mutations in DDR2 (Ali et al, 2010; Al-Kindi et al, 2014). In collaboration with Barbara Brodsky, Tufts University, USA, we defined an inhibitory collagen-like DDR ligand (An et al, 2016).
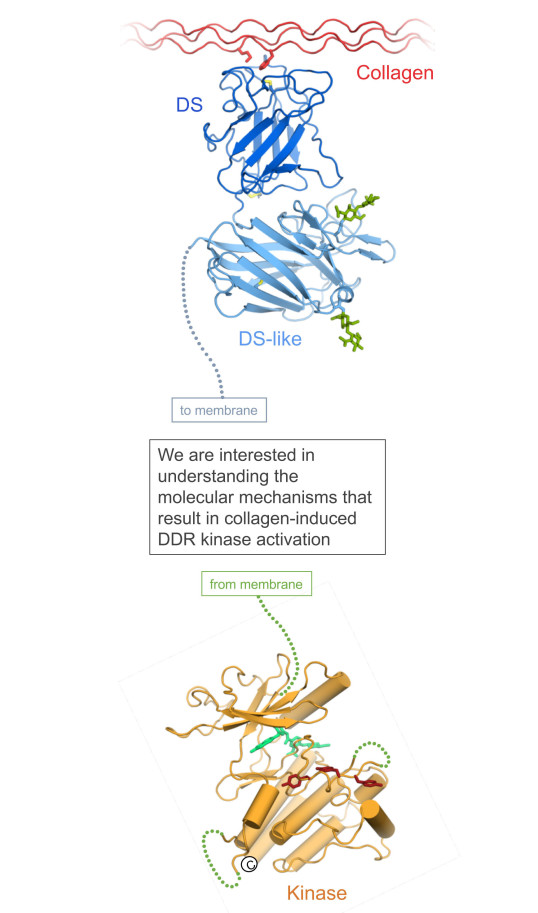
Key structural insight was gained through collaboration with Erhard Hohenester, Imperial College. A crystal structure of the DDR2 discoidin domain in complex with a triple-helical collagen-mimetic peptide defined the sequence-specific collagen recognition of the DDRs (Carafoli et al, 2009). Another crystal structure, in complex with an inhibitory anti-DDR1 antibody fragment (Carafoli et al, 2012) revealed the structure of the discoidin-like domain.
Recent work from our lab established phosphorylation between DDR1 dimers as a key mechanism contributing to DDR1 activation by collagen (Juskaite et al, 2017).
Guest Lectures
Collagen sensing: how discoidin domain receptors transmit a signal across the membrane and control kinase activity, Galapagos NV, Mechelen, Belgium (remote lecture), 2020
Discoidin domain receptors: multitaskers for physiological and pathological processes, 1st DDRs meeting, Bordeaux, France, 2019
Receptor tyrosine kinase activation: spotlight on collagen receptors in health and disease. Seminar Series,, Seminar Series, Cardiovascular and Cell Sciences Research Institute, Division, St George's University,, London, 2016
Collagen sensing: how do discoidin domain receptors transmit a signal across the membrane?, London Matrix Biology group Autumn Symposium, ‘Basement membranes: roles in Development and Disease’, William Harvey Research Institute, London, UK, 2015
How do discoidin domain receptors transmit a signal across the membrane?, Institute of Experimental Musculosceletal Medicine (IEMM), Westfälische Wilhelms University, Münster, Germany, 2015
How do discoidin domain receptors transmit a signal across the membrane?, Cardiovascular Division, King’s College London, London, UK, 2015
How do discoidin domain receptors transmit a signal across the membrane?, Roche Innovation Center, Basel, Switzerland, Basel, Switzerland, 2015
Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen, Gordon Research Conference Collagen, New London, NH, US, 2007
How do discoidin domain receptors transmit a signal across the membrane?, Matrix Biology Europe Conference, Rotterdam, the Netherlands, 2014
How do discoidin domain receptors transmit a signal across the membrane and modulate cell adhesion?, Center for Applied Medial Research, University of Navarra, Spain, 2012
Discoidin domain receptors: adhesion, collagen binding and transmembrane signaling, Merck Serono R&D, Darmstadt, Germany, 2012
Discoidin domain receptors: adhesion and transmembrane signaling. British Society for Matrix Biology, Matrix Signals! Cell-matrix interactions in health and Disease. Newcastle University, UK, 2011
Trafficking defects and loss of ligand binding are the underlying causes of all reported DDR2 missense mutations found in SMED-SL patients, XXIInd meeting of the Federation of the European Connective Tissue Societies, Davos, Switzerland, 2010
Trafficking defects and loss of ligand binding are the underlying causes of all reported DDR2 missense mutations found in SMED-SL patients, British Society for Matrix Biology, Vascular Matrix in Health and Disease, University of Manchester, UK, 2010
How discoidin domain receptors bind to collagen to transmit a signal across the membrane?, School of Biological Sciences, University of East Anglia, Norwich, UK, 2009
How discoidin domain receptors interact with collagen, 25th Ernst Klenk Symposium in Molecular Medicine - Extracellular matrix in Health and Disease, University of Cologne, Germany, 2009
How discoidin domain receptors interact with collagen, Gordon Research Conference Collagen, New London, NH, US, 2009
Collagen binding and transmembrane signalling by the discoidin domain receptors DDR1 and DDR2, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, US, 2009
How discoidin domain receptors interact with collagen, XXIst meeting of the Federation of the European Connective Tissue Societies, Marseille, France, 2008
Collagen binding and receptor activation of the discoidin domain receptors DDR1 and DDR2, Novartis Institute of Biomedical Research, Horsham, UK, 2007
Collagen binding and receptor activation of the discoidin domain receptors DDR1 and DDR2, London Matrix Group, University College London, UK, 2005
Discoidin domain receptors - receptor tyrosine kinases activated by collagen, The Cell Club, Imperial College London, 2005
Collagen binding and receptor activation of the discoidin domain receptors DDR1 and DDR2, Department of Biochemistry, University of Cambridge, UK, 2005
Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2: Identification of collagen binding sites in DDR2, 15th UK Adhesion Society Meeting, London, UK, 2003

