Summary
 Doryen Bubeck is a structural biologist who investigates host-pathogen interactions. She creates and applies model membranes systems to understand how information is relayed across membranes during infection and immunity. She received her PhD in Biophysics from Harvard University in 2005 where she used cryo-electron microscopy (cryo-EM) to understand the cell entry mechanism of poliovirus. As an EMBO postdoctoral fellow and Cancer Research Institute Fellow at the University of Oxford, she continued to explore the structures of membrane proteins, focusing on the complement immune pathway. Doryen joined Imperial College as a Lecturer in 2012 and with a Career Establishment Award from CRUK, she began her independent research career. She was promoted to Senior Lecturer in 2017, Reader in Structural Immunology in 2021 and Professor of Structural Immunology in 2023. She holds a satellite group leader position at the Francis Crick Institute since 2019 and is Director of the Centre for Structural Biology at Imperial College.
Doryen Bubeck is a structural biologist who investigates host-pathogen interactions. She creates and applies model membranes systems to understand how information is relayed across membranes during infection and immunity. She received her PhD in Biophysics from Harvard University in 2005 where she used cryo-electron microscopy (cryo-EM) to understand the cell entry mechanism of poliovirus. As an EMBO postdoctoral fellow and Cancer Research Institute Fellow at the University of Oxford, she continued to explore the structures of membrane proteins, focusing on the complement immune pathway. Doryen joined Imperial College as a Lecturer in 2012 and with a Career Establishment Award from CRUK, she began her independent research career. She was promoted to Senior Lecturer in 2017, Reader in Structural Immunology in 2021 and Professor of Structural Immunology in 2023. She holds a satellite group leader position at the Francis Crick Institute since 2019 and is Director of the Centre for Structural Biology at Imperial College.
Doryen's research group explores fundamental mechanisms in immunity and how pathogens hijack cellular pathways during infection. Her lab solved the structure of the complement membrane attack complex (MAC), a large protein immune pore that lyses cells. She discovered molecular drivers underpinning its sequential assembly mechanism and she explores how immune activation changes biophysical properties of the membrane to provide a general mechanism for how proteins cross lipid bilayers. Her recent work investigates how human cells stop MAC damage when the immune system is turned on. Her group has solved structures of inhibited MAC and now aims to explore their impact on cell signalling and membrane remodelling. Her structural models may then underpin the discovery of new therapeutics that regulate inflammatory and disease pathways.
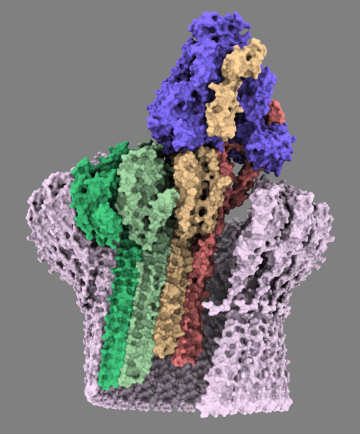
New work from the lab out in Nature Communications!

CD59 (pink) catches MAC assembly and stops pore formation
https://www.imperial.ac.uk/news/243212/research-shows-human-cells-protected-when/
Bubeck lab culture word cloud, created by lab members past and present:
Selected Publications
Journal Articles
Bubeck D, Couves E, Gardner S, et al., 2023, Structural basis for membrane attack complex inhibition by CD59, Nature Communications, Vol:14, ISSN:2041-1723, Pages:1-13
Shah NR, Voisin TB, Parsons ES, et al., 2020, Structural basis for tuning activity and membrane specificity of bacterial cytolysins, Nature Communications, Vol:11, ISSN:2041-1723
McFarlane C, Shah N, Kabasakal B, et al., 2019, Structural basis of light-induced redox regulation in the Calvin-Benson cycle in cyanobacteria, Proceedings of the National Academy of Sciences of the United States of America, Vol:116, ISSN:0027-8424, Pages:20984-20990
Parsons E, Stanley G, Pyne A, et al., 2019, Single-molecule kinetics of pore assembly by the membrane attack complex, Nature Communications, Vol:10, ISSN:2041-1723, Pages:1-10
Menny A, Serna M, Boyd C, et al., 2018, CryoEM reveals how the complement membrane attack complex ruptures lipid bilayers, Nature Communications, Vol:9, ISSN:2041-1723
Boyd CM, Bubeck DA, 2018, Advances in cryoEM and its impact on beta-pore forming proteins, Current Opinion in Structural Biology, Vol:52, ISSN:0959-440X, Pages:41-49
Boyd C, Parsons ES, Smith RAG, et al., 2016, Disentangling the roles of cholesterol and CD59 in intermedilysin pore formation, Scientific Reports, Vol:6, ISSN:2045-2322
Serna Gil M, Bubeck D, Giles JL, et al., 2016, Structural basis of complement membrane attack complex formation, Nature Communications, Vol:7, ISSN:2041-1723, Pages:1-7
Johnson S, Brooks NJ, Smith RAG, et al., 2013, Structural basis for recognition of the pore-forming toxin intermedilysin by human complement receptor CD59, Cell Reports, Vol:3
Hadders MA, Bubeck D, Roversi P, et al., 2012, Assembly and Regulation of the Membrane Attack Complex Based on Structures of C5b6 and sC5b9, Cell Reports, Vol:1, ISSN:2211-1247, Pages:200-207

