Summary
Dr. David Hodson completed a degree in Veterinary Science before undertaking doctoral studies in reproductive physiology and circadian biology at the University of Bristol under the supervision of Dr. Domingo Tortonese. Subsequently, in 2008, he moved to the laboratory of Dr. Patrice Mollard at the Institut de Genomique Fonctionnelle in Montpellier (CNRS), France, where, supported by a Fellowship from the Fondation de la Recherche Medicale (FRM), he pursued postdoctoral studies focused on understanding how endocrine cells residing within the mammalian pituitary gland function as three-dimensional networks to generate hormone release in response to demand. Following award of a Diabetes UK RD Lawrence Fellowship in 2012, David moved to the Section of Cell Biology at Imperial College London, before being recruited to the University of Birmingham in 2016. There, he is currently investigating how type 2 diabetes (T2D) insults target tissue- and organ-level processes to impact beta cell function and reduce insulin secretion.
research interests
Research in our lab revolves around the use of sophisticated imaging modalities (e.g. high-speed multibeam, two-photon, super-resolution), combined with recombinant technologies and genetic manipulation, to interrogate and decipher the population basis for hormone secretion from endocrine tissues in situ and in vivo. Focussing predominantly upon rodent and human islets of Langerhans, these studies have demonstrated a novel mode of incretin action which boosts insulin release through coaxing beta cell cooperativity, a role for GWAS-identified genes in regulating human beta cell-beta cell interconnectedness, and dual orchestration of metabolic dynamics in a subpopulation of beta cells by glucose and incretin.
On-going work concentrates on understanding: 1) how the three-dimensional islet microarchitecture influences the beta cell dynamics thought to underlie insulin secretion; 2) the role of tissue robustness and plasticity in determining cell susceptibility to the diabetic milieu; 3) species-heterogeneity in the structural and functional underpinnings of insulin secretion and how this may fail during T2D; and 4) the design and validation of novel tools and strategies with which to harness precise control over endocrine cells residing within their tissue context (photopharmacology and optogenetics).
Selected Publications
Journal Articles
Johnston NR, Mitchell RK, Haythorne E, et al., 2016, Beta cell hubs dictate pancreatic islet responses to glucose, Cell Metabolism, Vol:24, ISSN:1932-7420, Pages:389-401
Frank JA, Yushchenko DA, Hodson DJ, et al., 2016, Photoswitchable diacylglycerols enable optical control of protein kinase C., Nature Chemical Biology, Vol:12, ISSN:1552-4469, Pages:755-762
Broichhagen J, Johnston NR, von Ohlen Y, et al., 2016, Allosteric optical control of a class B G-protein-coupled receptor, Angewandte Chemie - International Edition, Vol:55, ISSN:1433-7851, Pages:5865-5868
Michau A, Hodson DJ, Fontanaud P, et al., 2015, Metabolism regulates exposure of pancreatic islets to circulating molecules in vivo., Diabetes, Vol:65, ISSN:0012-1797, Pages:463-475
Broichhagen J, Podewin T, Meyer-Berg H, et al., 2015, Optical control of insulin secretion using an incretin switch, Angewandte Chemie - International Edition, Vol:54, ISSN:1433-7851, Pages:15565-15569
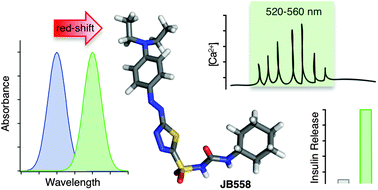
Broichhagen J, Frank JA, Johnston NR, et al., 2015, A red-shifted photochromic sulfonylurea for the remote control of pancreatic beta cell function, Chemical Communications, Vol:51, ISSN:1359-7345, Pages:6018-6021
Rutter GA, Broichhagen J, Schönberger M, et al., 2014, Optical control of insulin release using a photoswitchable sulfonylurea, Nature Communications, Vol:5, ISSN:2041-1723
Hodson DJ, Mitchell RK, Marselli L, et al., 2014, ADCY5 couples glucose to insulin secretion in human islets, Diabetes, Vol:63, ISSN:0012-1797, Pages:3009-3021
Schaeffer M, Langlet F, Lafont C, et al., 2013, Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons, Proceedings of the National Academy of Sciences of the United States of America, Vol:110, ISSN:0027-8424, Pages:1512-1517
Hodson DJ, Mitchell RK, Bellomo EA, et al., 2013, Lipotoxicity disrupts incretin-regulated human beta cell connectivity, Journal of Clinical Investigation, ISSN:1558-8238
Hodson DJ, Schaeffer M, Romano N, et al., 2012, Existence of long-lasting experience-dependent plasticity in endocrine cell networks, Nature Communications, Vol:3, ISSN:2041-1723
Sanchez-Cardenas C, Fontanaud P, He Z, et al., 2010, Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood, Proceedings of the National Academy of Sciences of the United States of America, Vol:107, ISSN:0027-8424, Pages:21878-21883
Lafont C, Desarmenien MG, Cassou M, et al., 2010, Cellular in vivo imaging reveals coordinated regulation of pituitary microcirculation and GH cell network function, Proceedings of the National Academy of Sciences of the United States of America, Vol:107, ISSN:0027-8424, Pages:4465-4470