Summary
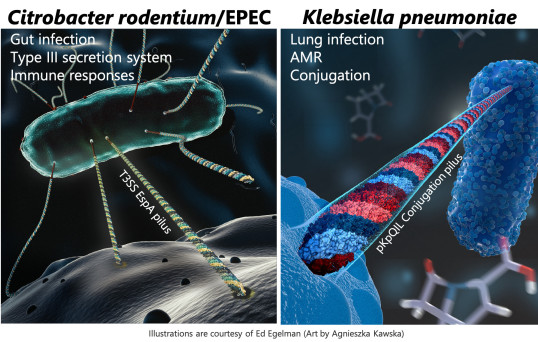
MOLECULAR PATHOGENESIS: GRAM-NEGATIVE BACTERIAL PATHOGENS AND AMR
We study infection of mucosal surfaces by enteric (Citrobacter rodentium) and pulmonary (Klebsiella pneumoniae) pathogens in the context of antimicrobial resistance, the microbiome and immunometabolism; and bacterial conjugation.
citrobacter rodentium and epec PATHOGENESIS AND HOST RESPONSES
Citrobacter rodentium is a mouse restricted pathogen that shares an infection strategy and virulence factors with the human pathogens enteropathogenic and enterohaemorrhatic E. coli (EPEC and EHEC). As mice are inherently resistant to EPEC and EHEC infection, C. rodentium provides a physiological model to study pathogenesis, immune responses, the role of microbiome and host genetics during infection and recovery.
Our Citrobacter rodentium projects include:
- The function and robustness of type III secretion system (T3SS) effector networks in vivo
- Adaptation of the host to pathogens using alternative T3SS effector networks
- Temporal changes and responses to infection in intestinal epithelial cells (IECs) using whole cellular proteomics
- The effect of host genetics on the outcome of bacterial infection
- Long-term recovery processes from infection, damage and recurrent perturbation of the colonic mucosa
EHEC, EPEC and C. rodentium colonise the gut mucosa via formation of attaching and effacing lesions.
EXTENSIVELY DRUG RESISTANT KLEBSIELLA PNEUMONIAE IN THE HUMAN LUNG
Klebsiella pneumoniae is an established human pathogen typically affecting hospitalised patients although an invasive community-acquired disease is also emerging in the far east. The rapid emergence of carbapenemase producing strains is the latest wave antimicrobial resistance mechanism among Gram-negative bacteria. In the United Kingdom, Klebsiella pneumoniae is now the most prevalent carbapenemase producing invasive isolate detected in patients. This project characterises large epidemic resistance plasmids in Klebsiella and their impact of virulence and persistence in mice to help understand the proliferation and spread of these resistance determinants. In vivo imaging is used to spatio-temporally track infection coupled with prokaryotic transcriptomics to identify novel targets for antimicrobial chemotherapy.
Bacterial Conjugation in Klebsiella pneumoniae
Conjugation is a process that is widely associated with the spread of antimicrobial resistance genes but many mechanistic details of plasmid transfer remain unknown. Using a carbapenemase encoding plasmid from Klebsiella pneumoniae, pKpQIL, we investigate the factors which affect conjugation efficiency. Fluorescence-based assays allow us to track plasmid transfer in both a high-throughput manner and in real time.
Group Members
Professor Gad Frankel
Gad Frankel obtained his B.Sc. in Biology and subsequently his Ph.D in Genetics from the Hebrew University of Jerusalem. He has held a Howard Hughes Fellowship in the Department Microbiology and Immunology, Stanford University, CA, USA. Following a short stay at the Weizmann Institute in Rehovot, he was appointed a research fellow at Imperial College. In 1998 he was appointed Lecturer at Imperial College, was promoted to Reader in 2000 and to Professor in 2002. He is currently Professor of Bacterial Pathogenesis at the Department of Life Sciences and the MRC Centre for Molecular Bacteriology and Infection (CMBI) at Imperial College London.
 |
 |
||
| Dr Vishwas Mishra | Dr Priyanka Biswas | Dr Rita Berkachy | Mr Jaie Rattle |
| Research Associate |
Research Associate |
Research Associate |
Research Assistant |
 |
 |
 |
|
| Mr Jonathan Bradshaw | Dr Julia Sanchez-Garrido | Dr Joshua L C Wong | Ms Shan He |
| PhD Student |
Research Associate |
Scientific Lead |
PhD Student |
 |
 |
 |
|
| Ms Sarah Jordan | Dr Karin Santoni | ||
| PhD Student |
PhD Student | Research Associate |
|
Selected Publications
Journal Articles
Cepeda-Molero M, Berger CN, Walsham ADS, et al., 2017, Attaching and effacing (A/E) lesion formation by enteropathogenic E. coli on human intestinal mucosa is dependent on non-LEE effectors, PLOS Pathogens, Vol:13, ISSN:1553-7366
Berger C, Crepin V, Roumeliotis TI, et al., 2017, Citrobacter rodentium subverts ATP flux 1 and cholesterol homeostasis in 2 intestinal epithelial cell in vivo, Cell Metabolism, Vol:26, ISSN:1550-4131, Pages:738-752.e6
Pearson JS, Giogha C, Ong SY, et al., 2013, A type III effector antagonizes death receptor signalling during bacterial gut infection, Nature, Vol:501, ISSN:0028-0836, Pages:247-+
Berger CN, Crepin VF, Baruch K, et al., 2012, EspZ of Enteropathogenic and Enterohemorrhagic Escherichia coli Regulates Type III Secretion System Protein Translocation, Mbio, Vol:3, ISSN:2161-2129



