Overview
Jan is part of the German Research Network on Neuropathic Pain (DFNS), and as such responsible for all questions related to statistical analyses of QST data within the European database, and was part of the European collaborations Neuropain and the IMI (Innovative Medicines Initiative) project Europain.
He is currently working on standardizing animal testing for more reproducible outcomes within the IMI project EQIPD. In collaboration with the Neurophysiology group of the Medical Faculty Mannheim in Germany within the IMI consortium PainCare he is pharmacologically validating functional pain biomarkers, and with partners at Harvard Medical School, Cambridge, US, working on determining predictors of placebo responses in clinical trials.
Quantitative Sensory Testing

Quantitative Sensory Testing (QST) as a means of assessing the psychophysical function of the peripheral sensory nervous system has been used to quantify both loss and gain of nervous function for decades. The German Research Network on Neuropathic Pain has developed a standardized protocol, that is by now accepted and used across Europe and applied on each continent (except Antarctica, to the best of our knowledge).
We could show that patients suffering from peripheral neuropathic pain present QST profiles that can be categorized into three phenotypes, characterized mainly by (a) sensory loss, (b) preserved sensation and thermal hyperalgesia, and (c) loss of small fibre function in combination with mechanical hyperalgesia.
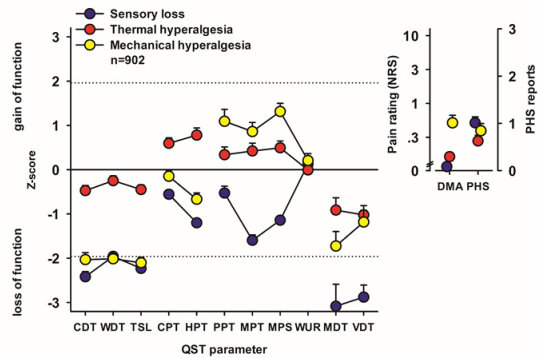
The European Medicines Agency has acknowledged that these profiles can be used as biomarkers for a mechanism-based stratification for future medication, enhancing the chance of effective, but selective new medication to reach the market in the future.
Preclinical Scientific Rigour

With the replication crisis looming over medical research, it is important to note that a failure of replicating results of a study must not be linked to an accusation of fortified results - the problem might rather be slight methodological differences, highly inbred animal strains used, and means of scientific conduct that amplify effects of the particular researcher on the outcome of their experiment, as for example in observer or confirmation bias.
To increase reproducibility and replicability of preclinical animal research, improving study design and reducing the risks of bias will be helpful.
Placebo effects in clinical trials

It has been debated if placebo effects in clinical trials are increasing. In clinical trials, a strong placebo response might lead to a negative outcome and to a promising new drug not reaching market. It would therefore be useful to understand and possibly predict the individual placebo response, as this could expose the full drug effect instead of relying on the additivity concept (effect - placebo effect = drug effect).
With colleagues from Harvard Medical School, Cambridge, US, and Aarhus University, Aarhus, Denmark, Jan works on meta analyses of multiple clinical trials to unravel placebo responses and how this information could inform future trial design.

