Summary
Prof Marina Kuimova received a M.Sc. in Chemistry from Moscow State University in 2001, and a Ph.D. from Nottingham University in 2005. Following a postdoc with Prof David Phillips and an EPSRC Life Science and Career Acceleration Fellowships, held at Imperial, she was appointed as a Lecturer in 2012 and was promoted to a Reader (Associate Professor) in 2016. She is currently a Professor in the Department of Chemistry.
RESEARCH
My research interests include elucidation of biologically relevant processes using different types of fluorescence imaging and time-resolved spectroscopy. The detailed description of my research activities can be found here
Our recent press release on Biophysical Journal paper can be found here.
For a bit of fun, read the NatureChem 'The Sceptical Chymist' blog entry here
OPPORTUNITIES
We are always looking to recruit talented and dedicated scientists. If you are interested in joining the group, please get in touch! It is likely that the funding for one or more PhD projects will be available for the 2022 start date.
Prospective PhD students please take note of
The President's PhD Scholarship Scheme
Chemistry Doctoral Scholarships scheme
The China Scholarship Council (CSC) Scheme
The Imperial Marshall Scholarship Scheme
The Schrodinger Scholarship Scheme
Funded positions will be advertised here when available
NEWS
*January 2021:
Our new paper on detecting G-quadruplex DNA in cells has been published in Nature Communications. Read the press release here.
*July 2019:
Miguel Paez-Perez won a poster prize at the 12th EBSA Congress in Madrid for his work entitled: Reorganization of lipid domains dampens changes in membrane tension.
Congratulations to Miguel!
*July 2019:
Welcome to two summer students who joined the lab as part of UROP: Solvejg Soll-Lauritzen and Jialin Li.
*May 2019:
Marina has been named as a winner of The Society of Porphyrins and Phthalocyanines Young Investigator Award, 2020. She will deliver her award lecture in Buffalo, NY in June next year at ICPP-11.
*May 2019:
Our new paper on cell viscosity under conditions of hypergravity has been published in Biophys J, read the press release about it here.
*Sep 2018:
Our new review on un-mixing of the temperature-viscosity sensitivity of molecular rotors has been published! A. Vysniauskas and M. K. Kuimova, A twisted tale: measuring viscosity and temperature of microenvironments using molecular rotors, International Reviews Phys Chem, 2018
*April 2018:
Our new paper An Optical Technique for Mapping Microviscosity Dynamics in Cellular Organelles has appeared in ASC Nano.
Congratulations to the joint first authors Joe and Marketa!
Also read about Surface functionalisation with viscosity-sensitive BODIPY molecular rotor, published in MAF journal
Congratulations to Aurimas and Ismael, with excellent support from Alex!
*October-November 2017:
Welcome to the new people joining the group: Jeff Lam (MSci), Miguel Paez Perez (PhD), Dr Peter Summers, Dr Raju Laishram and Dr Ajesh Thomas!
*July 2017:
Marketa is one of the winners of an EPSRC Doctoral Prize Fellowship, which she will start in the group on the 1st October. Congratulations to Marketa!
*July 2017:
Our new paper entitled 'Exploring viscosity, polarity and temperature sensitivity of BODIPY-based molecular rotors' has appeared on the cover of PCCP. Here we explore the effect of temperature on several BODIPY molecular rotors, with somewhat unexpected results!
Our study of the effect of temperature on the functioning of porphyrin dimer-based molecular rotors is published here: 'Tuning the Sensitivity of Fluorescent Porphyrin Dimers to Viscosity and Temperature'. Please check our frontispiece of Chem Eur J.
Congratulations to Aurimas (lead author) on both papers and an excellent support team from the group: Dong, Maryam and Ismael!
*June 2017:
Our new paper entitled 'Probing supramolecular protein assembly using covalently attached fluorescent molecular rotors' has appeared in Biomaterials. Here we studied the aggregation of Amyloid Beta and other aggregating proteins using covalently attached molecular rotors.
Congratulations to Marketa!
*March 2017:
Our bid for Imperial Excellence Fund for Frontier Research in collaboration with Prof Ramon Vilar and Dr Jean-Baptiste Vannier was successful! Read about 'Searching for DNA quadruplexes' here.
SELECTED PUBLICATIONS
A. Vysniauskas and M. K. Kuimova, A twisted tale: measuring viscosity and temperature of microenvironments using molecular rotors, International Reviews Phys Chem, 2018 A REVIEW!
J. E. Chambers, M. Kubankova et al, An Optical Technique for Mapping Microviscosity Dynamics in Cellular Organelles ASC Nano, 2018, 12 (5), 4398-4407
P. Sherin et al, Visualising the membrane viscosity of porcine eye lens cells using molecular rotors, Chem Sci 2017
M. Kubankova et al, 'Probing supramolecular protein assembly using covalently attached fluorescent molecular rotors', Biomaterials 2017.
L. E. Shimolina et al, Imaging tumor microscopic viscosity in vivo using molecular rotors, Sci Reports 2017
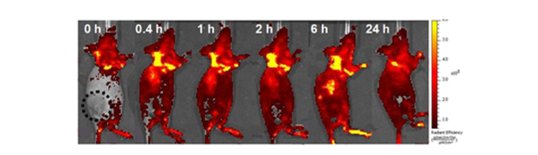
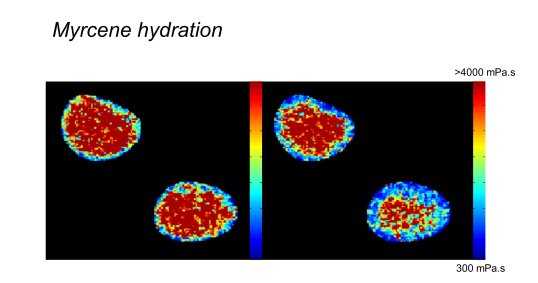
N. A. Hosny et al Direct imaging of changes in aerosol particle viscosity upon hydration and chemical aging, Chem. Sci., 2016, 7, 1357-1367
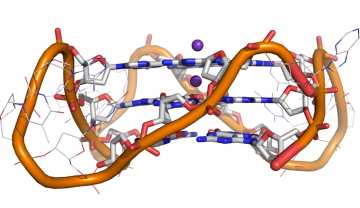
A. Shivalingam, et al The interactions between a small molecule and G-quadruplexes are visualised by fluorescence lifetime imaging microscopy. Nature Commun., 2015, 6, 8178
A. Vysniauskas et al Unravelling the effect of temperature on viscosity-sensitive fluorescent molecular rotors, Chem Sci., 2015, DOI: 10.1039/C5SC02248G
I. Lopez-Duarte, T. T. Vu, et al A molecular rotor for measuring viscosity in plasma membranes of live cells, Chem. Commun., 2014, DOI: 10.1039/C3CC47530A (Emerging investigators issue)
Neveen A. Hosny et al Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors, PNAS, 2013
M.K. Kuimova Mapping viscosity in cells using molecular rotors, PCCP, 2012, 14, 12671-12686 A REVIEW!
M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling, P. R. Ogilby, Imaging Intracellular Viscosity of a Single Cell During Photoinduced Cell Death, Nature Chem., 2009, 1, 69
Publications
Journals
Paez Perez M, Dent MR, Brooks N, et al., 2023, Directly imaging emergence of phase separation in peroxidized lipid membranes, Communications Chemistry, ISSN:2399-3669
Sherin PS, Vysniauskas A, Lopez-Duarte I, et al., 2021, Visualising UV-A light-induced damage to plasma membranes of eye lens, Journal of Photochemistry and Photobiology, B: Biology, Vol:225, ISSN:1011-1344, Pages:1-10
Summers PA, Thomas AP, Kench T, et al., 2021, Cationic helicenes as selective G4 DNA binders and optical probes for cellular imaging, Chemical Science, Vol:12, ISSN:2041-6520, Pages:14624-14634
Vilar R, Priessner M, Summers PA, et al., 2021, Selective detection of Cu+ ions in live cells via fluorescence lifetime imaging microscopy., Angewandte Chemie International Edition, Vol:60, ISSN:1433-7851, Pages:23148-23153
Shimolina L, Lukina M, Shcheslavskiy V, et al., 2021, Probing metabolism and viscosity of cancer cells using fluorescence lifetime imaging microscopy, Jove-journal of Visualized Experiments, ISSN:1940-087X, Pages:1-22

