Overview
Our current work uses variety of imaging and spectroscopic techniques to elucidate the nature of processes involved in cell function and death, including those during Photodynamic Therapy (PDT) treatment of cancer and amyloidogenic diseases.
Our method of choice is Fluorescence Lifetime Imaging Microscopy (FLIM).We design and characterise FLIM probes of viscosity, temperature, polarity and unusual DNA conformations such as DNA G quadruplexes.
My research interests also include ultrafast processes involving excited states of biomolecules, such as DNA, and photophysics of coordination compounds.
The popular article and the video clip where I talk to the media can be found here
We use different types of fluorescence imaging and time-resolved spectroscopy to investigate several themes outlined below.
The group
Clockwise: Dr Raju Laishram, Jeff Lam, Dr Ajesh Thomas,Dr Peter Summers, Dr Marina Kuimova, Ben Lewis, Marketa Kubankova, Miguel Paez Perez
Molecular rotors
Molecular rotors are small fluorophores in which fluorescence competes with intramolecular rotation. In a viscous environment rotation is slowed down and this strongly affects fluorescence parameters, such as intensity, decay time and spectral profile. We have designed rotors which allow to measure viscosity by changes in their fluorescence spectra or lifetimes. We have studied the effect of temperature and polarity on molecular rotors and used them in a variety of applications.
A. Vysniauskas and M. K. Kuimova, A twisted tale: measuring viscosity and temperature of microenvironments using molecular rotors, International Reviews Phys Chem, 2018
M. K. Kuimova, G. Yahioglu, J. A. Levitt, K. Suhling, Molecular Rotor Measures Viscosity of Live Cells via Fluorescence Lifetime Imaging, J. Amer. Chem. Soc., 2008, 130, 6672
M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling, P. R. Ogilby, Imaging Intracellular Viscosity of a Single Cell During Photoinduced Cell Death, Nature Chem., 2009, 1, 69
IMAGING DNA G-QUADRUPLEXES (G4s)
Guanine rich oligonucleotides can fold into secondary structures known as G-quadruplexes, which are proposed to have various biological roles, however, their visualisation in live cells still remains a challenge. We are using Fluorescence Lifetime Imaging microscopy to quantitatively and dynamically image the presence of G4s inside live cells.
Our recently discovered cell-permeable, low-toxicity sensor enabled the quantification and dynamic monitoring of this rare DNA topology in live cells for the first time. This work is in close collaboration with the groups of Prof Ramon Vilar and Jean Baptiste Vannier.
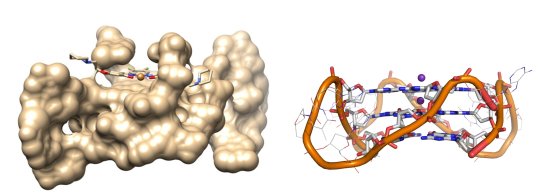
A. Shivalingam et al, The interactions between a small molecule and G-quadruplexes are visualized by fluorescence lifetime imaging microscopy, Nature Communications volume 6, Article number: 8178 (2015) |
Imaging atmospheric aerosols
Organic aerosol particles play major roles in atmospheric chemistry, climate, and public health. There is recent evidence that they might be present in highly viscous states, however, direct observational evidence is lacking, as are methods that are able to dynamically quantify and track viscosity changes during atmospherically relevant processes. We are using molecular rotors to provide quantitative real-time, online observation of aerosol microscopic viscosity in atmospherically relevant aerosol particles. Such data cannot be obtained with any other currently used technique. We have designed and characterised molecular rotors suitable for the particularly high viscosity ranges needed for aerosol mapping.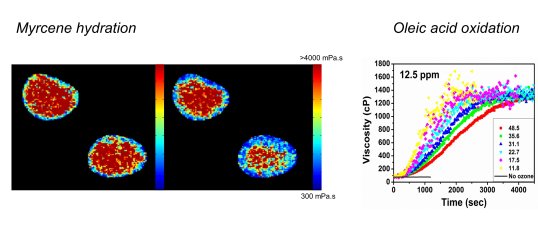
N. A. Hosny et al, 'Direct imaging of changes in aerosol particle viscosity upon hydration and chemical aging' Chemical Science 2015.
A. Athanasiadis et al, Dynamic viscosity mapping of the oxidation of squalene aerosol particles, PCCP, 2016
C. Fitzgerald, Fluorescence lifetime imaging of optically levitated aerosol: a technique to quantitatively map the viscosity of suspended aerosol particles, PCCP 2016
Photodynamic Therapy (PDT)
PDT is a clinically tested promising technique to treat cancer. PDT uses red light to activate light-sensitive drugs (photosensitizers) to produce short lived cytotoxic species such as singlet oxygen to destroy malignant cells. We investigate mechanisms of cell death during PDT using fluorescence microscopy and imaging and perform spectroscopic measurements to help design better photosensitizers
M. K. Kuimova, H. A. Collins, M. Balaz, E. Dahlstedt, J. A. Levitt, N. Sergent, K. Suhling, M. Drobizhev, N. S. Makarov, A. Rebane, H. L. Anderson and D. Phillips, Photophysical properties and intracellular imaging of water-soluble porphyrin dimers for two-photon excited photodynamic therapy, Org. Biomol. Chem., 2009, 7, 889
M. K. Kuimova, M. Bhatti, M. Deonarain, G Yahioglu, J. A. Levitt, I. Stamati, K. Suhling, D. Phillips, Fluorescence characterisation of multiply-loaded anti-HER2 single chain Fv-photosesitizer conjugates suitable for photodynamic therapy, Photochem. Photobiol. Sci., 2007, 6, 933
M. K. Kuimova, M. Balaz, H. L. Anderson, P. R. Ogilby, Intramolecular rotation in a porphyrin dimer controls singlet oxygen production, J. Amer. Chem. S oc., 2009, 131 (23), 7948
Measuring intracellular singlet oxygen
Singlet oxygen is the main cytotoxic agent in PDT. Since the lifetime and reactivity of this species is d etermined to a large extent by diffusion, monitoring singlet oxygen in a cell provides us with a sensitive probe of viscosity.
M. K. Kuimova, G. Yahioglu and P. R. Ogilby, Singlet Oxygen in a Cell: Spatially-Dependent Lifetimes and Quenching Rate Constants, J. Amer. Chem. Soc., 2009, 131 (1), 332
M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling, P. R. Ogilby, Imaging Intracellular Viscosity of a Single Cell During Photoinduced Cell Death, Nature Chem., 2009, 1, 69
Monitoring DNA photodamage with IR spectroscopy
Light induced DNA damage is a constant threat to the stability of the genetic code. An important portion of DNA damage stems from radical products of ionization/oxidation attack that lead to irreversible defects, such as double strand breaks. We use picosecond time-resolved and steady-state infrared spectroscopy to monitor the crucial intermediates in these processes: radical cations of DNA bases as well as secondary products such as neutral radicals and 8-hydroxy-2''''-deoxyguanosine (8-oxo-G)
W. Parker, C.Y. Lin, M. W. George, M. Towrie and M. K. Kuimova Infrared Characterization of the Guanine Radical Cation: Finger Printing DNA Damage. J. Phys. Chem. B., 2010, 114, 3660–3667
M. K. Kuimova, P. M. W. Gill, C.-Y. Lin, P. Matousek, M. Towrie, X-Z Sun, M. W. George, A. W. Parker, Picosecond time-resolved infrared study of 2-aminopurine ionisation in Solution, Photochem. Photobiol. Sci., 2007, 6, 949
M. K. Kuimova, A. J. Cowan, P. Matousek, A. W. Parker, X. Z. Sun, M. Towrie and M. W. George, Monitoring the direct and indirect damage of DNA bases and polynucleotides by using time-resolved infrared spectroscopy, Proc. Nat. Acad. Sci. USA, 2006, 103, 2150
The popular article and the video clip where I talk to the media can be found here.
Invited lectures
17th Methods and Applications in Fluorescence (MAF17) conference, September 2020, Gothenburg, Sweden, (plenary).
International Conference on Porphyrins and Phthalocyanines (ICPP-11), Buffalo, June 2020, Young Investigator Award lecture
ESP-IUPB World Congress on Light and Life, Barcelona, August 2019
University of Amsterdam, Netherlands, June 2019
Peter Timms Symposium (Bristol), June 2019 (keynote)
12th Young Scientist Symposium, Bordeaux, May 2019 (keynote)
School of Engineering and Material Sciences, Queen Mary, University of London, May 2019
Chemistry Department, Nottingham University, February 2019
3rd Strasbourg Workshop on Membrane Biophysics, Strasbourg, December 2018
Sensors Day, Cambridge, October 2017
Chemistry Department, Leicester University, November 2017
15th Methods and Applications in Fluorescence (MAF2015) conference, Bruges, Belgium, September 2017
Gordon Research Conference on Artificial Molecular Switches and Motors", Holderness, NH (USA), June 2017
Materials and Metallurgy Department, Cambridge University, June 2017
Frankland symposium, Imperial College London, June 2017
Chemistry Department, Tallinn University, May 2017
International symposium at the Institute of Transformative Bio-Molecules (ITbM), Nagoya, Japan, December 2016
International Conference of Porphyrins and Phthalocyanines (ICPP9), Nanjing, China, July 2016
11th Workshop and Conference on Advanced Multiphoton and Fluorescence Lifetime Imaging Techniques, Vestec Czech Republic, June 2016
London Centre for Nanotechnology, May 2016
London Chemical Biology Colloquium, London June 2015
EMBO practical course on Single Molecule and Single Cell Fluorescence, Heidelberg, March 2015
Department of Chemistry, Aarhus University, Denmark, August 2014
25th IUPAC Symposium on Photochemistry, Bordeaux, France, July 2014
Universidad del País Vasco-EHU, Bilbao, Spain, July 2014
Donostia International Physics Center (DIPC), San Sebastian, Spain, July 2014
8th IUPAP International Conference on Biological Physics, Beijing, China, June 2014
American Society for Photobiology Meeting, San Diego, June 2014
Chemistry Department, Cardiff University March 2014
Chemistry Department, The University of East Anglia, March 2014
Université Pierre et Marie Curie, Laboratoire Jean Perrin, Paris, February 2014
Chemistry and Biochemistry and Cell and Molecular Biology, Queen Mary, University of London, January 2014
Cardiff School of Biosciences, October 2013
15th European Society for Photobiology Congress (ESP Congress), Liege, Belgium, September 2013
26th International Conference on Photochemistry (ICP), Leuven, Belgium, July 2013
Chemistry Department, Manchester University, May 2013
Department of Pharmacy and Pharmacology, University of Bath, April 2013
Joint Meeting of the British and German Biophysical Societies, Hünfeld, Germany, March 2013
Chemistry Department, Durham University, March 2013
The Institute of Pharmaceutical Science, King’s College London, February 2013
RSC Faraday Symposium (Harrison-Meldola Prize lecture), Department of Chemistry, Imperial College, UK (2012)
RSC Faraday Symposium (Harrison-Meldola Prize lecture), Department of Chemistry, UCL, London, UK (2012)
Coherent Multiphoton Conference 2012: Frontiers in Bioimaging, London, UK (2012)
British Biophysical Society Young Investigator Medal Lecture, (BBS Annual meeting), Durham, UK (2012)
Chemistry Department, Cambridge University, UK (2012)
Bristol Centre for Functional Nanomaterials, Bristol University, UK (2012)
Heyrovsky Institute of Physical Chemistry, Academy of Sciences of the Czech Republic (2012)
Chemistry Department, Oxford University, UK (2012)
Department of Chemistry, University of Newcastle, UK (2011)
VI Congress of the Russian Society for Photobiology, Tuapse, Russia (2011)
Grammaticakis-Neumann Prize Lecture, Swiss Chemical Society Fall Meeting, EPFL, Switzerland (2011)
PSIBS, University of Birmingtham, UK (2011)
Physics Department, University of Surrey, UK (2011)
London Centre for Nanotechnology, Imperial College London, UK (2011)
Department of Chemistry, UCL, UK (2010)
Department of Chemistry and Biochemistry, Georgia Institute of Technology, USA (2010)
Department of Biochemistry and Biophysics, University of Pensylvannia, USA (2010)
Department of Chemistry, Edinburgh University, UK (2009)
Department of Chemistry, University of Wyoming, USA (2009)
COMI, Aarhus University, Denmark (2007)
Division of Surgical Sciences, UCL, UK (2007)
Past group pictures (Nov 2014)
Left to right: Thanos Athanasiadis, Dr Rob Richardson, Dr Ismael Lopez Duarte, Aurimas Vysniauskas, Dr Alex Thompson, Michael Dent, Dr Marina Kuimova
Front: Dr Neveen Hosny, Maryam Qurashi, Dr Maria Izquierdo, Dr Thanh Vu, Marketa Kubankova
December 2012
Left to right: Michael Dent, Dr Neveen Hosny, Dr Ismael Lopez Duarte, Aurimas Vysniauskas, Dr Alex Thompson, Dr Marina Kuimova Front: Dr Thanh Vu, Maryam Qurashi, Dr Maria Izquierdo
May 2011
Left to right: Dr Changlun Tong, Maryam Qurashi, Yilei Wu, Dr Marina Kuimova, Dr Neveen Hosny, Will Mason

