Summary
Prof. Edison is pleased to consider applications from prospective PhD students.
Prof. Edison is the Professor of Neuroscience and Clinical Professor in the Department of Brain Sciences in the Faculty of Medicine at Imperial College London and an honorary Professor at Cardiff University, Wales. He is the Editor-in-Chief of the journal BRAIN CONNECTIVITY, an associate editor for the Journal of Alzheimer’s Disease, and serves in the editorial boards of the Journal of Neuroimaging and Contrast Media & Molecular Imaging. He is also a Consultant at Hammersmith Hospital, London.
Dr. Edison, after his clinical training (MD), did his MPhil and PhD at Imperial College London, and then completed his higher training in London Deanery and obtained his CCT from the Postgraduate Medical Education and Training Board. He then became a Fellow of Royal College of Physicians, Ireland and Fellow of Royal College of Physicians, UK.
He has received prestigious MRC clinical research fellowship award, HEFCE Clinical Senior Lectureship Award, and research scholarships.
His research is focused on neuroimaging with novel molecular probes using PET and magnetic resonance techniques for imaging pathophysiological changes associated with Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative diseases. He has extensive experience in PET and MR imaging in different neurodegenerative and neuroinflammatory conditions. His initial work evaluated one of the first amyloid imaging agent in Alzheimer’s disease at the MRC Cyclotron Unit, Imperial College London, Hammersmith Hospital, and has published seminal papers in the field. Since then, combined with his clinical expertise in different types of degenerative diseases and dementia, he has investigated the relationship between amyloid deposition, microglial activation, glucose metabolism, and MRI changes in different disorders, along with evaluating different transporters in the brain.
His work now focuses on neuroinflammation, and the interplay between inflammation and immunity in neurodegenerative and neuroinflammatory disease and relating these with genetic information. He is also evaluating the methods of modulating inflammation and amyloid in Alzheimer’s disease, and the influence of cardiometabolic factors on the development of neurodegenerative diseases by means of clinical and pre-clinical studies.
He has published in high impact journals such as Brain, Annals of Neurology, and Neurology, and has received grants from the Medical Research Council, NIHR/HEFCE, Alzheimer’s Society, Alzheimer’s Research UK, Alzheimer’s Drug Discovery Foundation US, and other funders. He collaborates closely with Novo Nordisk, GE Healthcare, Novartis, Piramal Life Sciences, and Astra Zeneca. He has also received several best paper awards internationally.
He is the chief investigator for several multicentre studies, one of which is evaluating Liraglutide in treatment of Alzheimer's disease, which is conducted in 24 sites.
He leads the Imperial College Memory Research centre and is the Chief Investigator of several other imaging studies using PET and MRI, and heads multicentre studies evaluating novel treatment of Alzheimer's and other neurodegenerative diseases. He also runs a memory clinic at Imperial College Healthcare NHS Trust.
IMPERIAL COLLEGE MEMORY RESEARCH CENTRE
At the Imperial College Memory Research Centre led by Dr Paul Edison, we focus our research on Alzheimer's disease, mild cognitive impairment, and causes of dementia and cognitive impairment. We evaluate neuroinflammation and neurodegeneration using PET, MRI, and CSF markers. We are now evaluating the relationship between
neuroinflammation, tau, glucose metabolism, and structural and functional changes measured by MRI. We heavily focus on basic and translational imaging using PET and MRI. We are evaluating novel therapeutic targets in dementia and cognitive impairment in Phase 1 and Phase 2/3 studies. We are evaluating novel microglial agents in mild cognitive impairment and Alzheimer's disease.
Additionally the unit uses state of the art techniques in PET and MRI in clinical evaluation of people with memory problems.
LIRAGLUTIDE IN TREATMENT OF ALZHEIMER's disease (ELAD)
There is a connection between type 2 diabetes and Alzheimer's disease; people with type 2 diabetes are much more likely to develop Alzheimer's disease than healthy people of the same age group.
The GLP-1 incretin analogue, liraglutide, is licensed and is being used safely in patients with type 2 diabetes mellitus. Experiments in AD transgenic mousemodels have shown that liraglutide crosses the blood-brain barrier, protects cognition, reduces amyloid plaqueformation, reduces inflammatory response, reduces levels of soluble amyloid oligomers, normalises synapticplasticity in the hippocampus, prevents progressive deterioration in glucose metabolism in the brain andincreases the proliferation of neuronal progenitor cells and the number of new neurons in the dentate gyrus. Liraglutide has demonstrated significant neuroprotective effect in preclinical studies and in a proof of concept PET study.
This is a 12-month, multicentre, randomised, double-blind, placebo-controlled Phase IIb study in patients with mild Alzheimer’s disease (AD) led and sponsored by Imperial College London. 206 patients will be randomised on a 1:1 ratio to receive liraglutide (1.8 mg daily by subcutaneous injection) or matching placebo from 20 centres around the UK and Ireland. This study aims to investigate the effect of liraglutide on glucose metabolism, MRI volume changes, amyloid load, tau formation and neuroinflammation using PET and MRI scans. Subjects will have brain scans at baseline and after 12 months of treatment. All scans are done at Imperial College Clinical Imaging Facility
We are leading a multicentre clinical trial between 24 UK research sites, including King's College London, University of Cambridge, University of Southampton, University of Bristol, Birmingham University, University of Brighton, St Georges University of London evaluating the novel diabetic drug, liraglutide in the treatment of Alzheimer's disease (ELAD study). Liraglutide is currently licensed for diabetes, and has already passed through all the aspects of drug development.
To take part in any of our studies please contact us on
e-mail: memory@imperial.ac.uk or memory@imperial.nhs.uk
Tel: 020 8383 3704; 020 8383 1969
Imaging amyloid and neuroinflammation in subjects at risk for Alzheimer's disease
Neuroinflammation has been proposed to be a link between amyloid deposition, damage to neurons and formation of “tangles”. It has been shown that inflammation takes place with the deposition of amyloid in animals, but the relative timings have not been studied in humans. In this study, we are performing PET brain scans in 140 subjects using [11C]PBR28, a PET marker of neuroinflammation, while [18F]flutemetamol is used to evaluate deposition and [18F]AV1451 is a marker of tau deposition.
Evaluation of microglial activation in Alzheimer’s disease and mild cognitive impairment subjects using a novel TSPO marker, GE180
[18F]GE180 is a novel PET imaging agent that has been developed for the assessment of neuroinflammation. TSPO levels in the normal central nervous system are very low, but increase dramatically in microglial cells in response to brain damage and inflammation. The TSPO tracer, [11C](R)PK11195, has been used for over 20 years to image brain inflammation. However, [11C]PK11195 has a very low specific to non-specific binding ratio and short radioactive half-life, making it a less than optimal imaging agent. In this study we are evaluating the novel tracer, [18F]GE180, in AD and other neurodegenerative diseases.
IMAGING AMYLOID AND NEUROINFLAMMATION IN SUBJECTS AT RISK FOR ALZHEIMER'S DISEASE
With PET we have previously shown that Alzheimer’s disease (AD) subjects have significantly increased accumulation of β-amyloid in the brain. In this research, we propose a proof-of-concept study to characterise the brain uptake of a novel astroglial activation imaging marker, [11C]BU99008, in a population of patients with mild to moderate AD. We will define the relationships between brain uptake of this marker, amyloid deposition (measured with [18F]florbetaben) and cerebral glucose metabolism (measured by uptake of [18F]FDG).
Evaluating microglial activation and reduced glucose metabolism in later stages of neurodegenarative diseases
Genetic and post-mortem studies have highlighted an active role of neuroinflammation in the neuropathology of neurodegenerative diseases. The aim of this study is to assess whether neuroinflammation and related neuronal function in neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease.
An in vivo evaluation of role of amyloid in delirium secondary to infection
Patients who develop delirium have higher morbidity, mortality, institutionalisation, along with longer in-patient stay than non-delirious patients. Delirium is strongly associated with infection and other inflammatory processes in which pro-inflammatory cytokines play a role. Experimental evidence suggests that infection leads to increased production of pro-inflammatory cytokines and this could lead to delirium and accelerated neurodegeneration. In this study, we are evaluating the role of amyloid in delirium.
Evaluation of caspase activation in Alzheimer’s disease using [18F]ICMT11 PET/CT
The memory problems caused by Alzheimer’s disease are associated with high levels of abnormal proteins in the brain, one of which is β-amyloid. Build-up of amyloid proteins and high levels of the enzyme, caspase, are linked with neuronal damage and cell death in the brain. This study evaluates the novel PET radiotracer, [18F]ICMT11, which specifically highlights the parts of brain with high caspase levels. In this study we aim to look at caspase activation in the brain using the radiotracer [18F]ICMT11. We will also use MRI to compare with the PET scan
COMMERCIAL STUDIES
A 24-month, multicenter, randomised, double-blind, placebo-controlled, parallel-group, efficacy, safety, tolerability, biomarker, and pharmacokinetic study of AZD3293 in early Alzheimer’s disease
Alzheimer’s disease (AD) is a progressive and fatal disorder of the nervous system, which results in amyloid plaque formation in specific regions of the brain, which is associated with inflammation and loss of neurones and synapses. AD is characterised by memory loss and progressively impairs activities of daily living.
Beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) is involved in the formation of amyloid plaques in AD. BACE1 is therefore considered to be a promising therapeutic target for slowing down or halting disease progression in AD. BACE1 splits amyloid precursor protein (APP) into a group of peptide fragments, which are the main component of the amyloid plaques. The study drug, AZD3293, is an inhibitor of BACE1 and has been shown to reduce peptide fragments in mice, rats, guinea pigs, dogs and humans. The study is being carried out to test whether AZD3293 modifies the clinical course of AD by delaying disease progression in patients diagnosed with early AD.
Selected Publications
Journal Articles
Leng F, Hinz R, Gentleman S, et al., 2023, Neuroinflammation is independently associated with brain network dysfunction in Alzheimer's disease, Molecular Psychiatry, Vol:28, ISSN:1359-4184, Pages:1303-1311
Nowell J, Blunt E, Edison P, 2023, Incretin and insulin signaling as novel therapeutic targets for Alzheimer's and Parkinson's disease, Molecular Psychiatry, Vol:28, ISSN:1359-4184, Pages:217-229
Pasqualetti G, Thayanandan T, Edison P, 2022, Influence of genetic and cardiometabolic risk factors in Alzheimer?s disease, Ageing Research Reviews, Vol:81, ISSN:1568-1637
Young M, Crook H, Scott J, et al., 2022, Covid-19: virology, variants, and vaccines, Bmj Medicine, Vol:1, ISSN:2754-0413
Livingston NR, Calsolaro V, Hinz R, et al., 2022, Relationship between astrocyte reactivity, using novel <SUP>11</SUP>C-BU99008 PET, and glucose metabolism, grey matter volume and amyloid load in cognitively impaired individuals, Molecular Psychiatry, Vol:27, ISSN:1359-4184, Pages:2019-2029
Pascoal TA, Benedet AL, Ashton NJ, et al., 2021, Microglial activation and tau propagate jointly across Braak stages, Nature Medicine, Vol:27, ISSN:1078-8956, Pages:1592-+
Crook H, Raza S, Nowell J, et al., 2021, Long covid-mechanisms, risk factors, and management., Bmj, Vol:374, ISSN:1759-2151, Pages:1-18
Calsolaro V, Matthews PM, Donat CK, et al., 2021, Astrocyte reactivity with late onset cognitive impairment assessed in-vivo using 11C-BU99008 PET and its relationship with amyloid load, Molecular Psychiatry, Vol:26, ISSN:1359-4184, Pages:5848-5855
Edison P, 2021, Microglial activation and blood-brain barrier leakage: chicken and egg?, Brain, Vol:144, Pages:1284-1285
Leng F, Edison P, 2021, Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here?, Nat Rev Neurol, Vol:17, Pages:157-172
Femminella GD, Livingston NR, Raza S, et al., 2021, Does insulin resistance influence neurodegeneration in non-diabetic Alzheimer's subjects?, Alzheimers Research & Therapy, Vol:13, ISSN:1758-9193, Pages:1-11
Femminella GD, Harold D, Scott J, et al., 2021, The Differential Influence of Immune, Endocytotic, and Lipid Metabolism Genes on Amyloid Deposition and Neurodegeneration in Subjects at Risk of Alzheimer’s Disease, Journal of Alzheimers Disease, Vol:79, ISSN:1387-2877, Pages:127-139
Leng F, Edison P, 2021, Neuroinflammation and microglial activation in Alzheimer’s disease: where do we go from here?, Nature Rev Neurol
Edison P, 2020, Neuroinflammation, microglial activation, and glucose metabolism in neurodegenerative diseases, International Review of Neurobiology, Vol:154, ISSN:0074-7742, Pages:325-344
Femminella G, Dani M, Wood M, et al., 2019, Microglial activation in early Alzheimer trajectory is associated with higher grey matter volume, Neurology, Vol:92, ISSN:1526-632X, Pages:e1331-e1343
Femminella GD, Taylor-Davies G, Scott J, et al., 2018, Do Cardiometabolic Risk Factors Influence Amyloid, Tau, and Neuronal Function in APOE4 Carriers and Non-Carriers in Alzheimer's Disease Trajectory?, Journal of Alzheimers Disease, Vol:64, ISSN:1387-2877, Pages:981-993
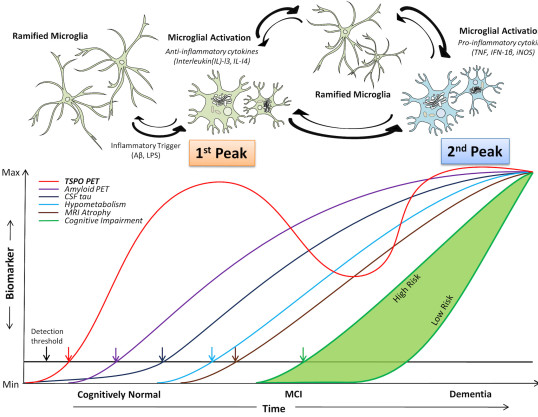
Fan Z, Brooks DJ, Okello A, et al., 2017, An early and late peak in microglial activation in Alzheimer's disease trajectory, Brain, Vol:140, ISSN:0006-8950, Pages:792-803
Fan Z, Okello AA, Brooks DJ, et al., 2015, Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease, Brain, Vol:138, ISSN:1460-2156, Pages:3685-3698
Fan Z, Aman Y, Ahmed I, et al., 2015, Influence of microglial activation on neuronal function in Alzheimer's and Parkinson's disease dementia, Alzheimers & Dementia, Vol:11, ISSN:1552-5260, Pages:608-621
Ashraf A, Fan Z, Brooks DJ, et al., 2014, Cortical hypermetabolism in MCI subjects: a compensatory mechanism? Eur J Nucl Med Mol Imaging (in press)., Eur J Nucl Med Mol Imaging
Edison P, Ahmed I, Fan Z, et al., 2013, Microglia, Amyloid, and Glucose Metabolism in Parkinson’sDisease with and without Dementia, Neuropsychopharmacology

