Imperial College Memory Research Centre
Prof. Edison is the Professor of Neuroscience in the department of Brain Sciences at Imperial College London and an honorary Professor at Cardiff University, Wales. He is also a Consultant Physician at Hammersmith Hospital, London.
Prof. Edison’s research has focused on neuroimaging with novel molecular probes using PET and magnetic resonance techniques for imaging pathophysiological changes associated with Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative diseases. He has extensive experience in PET imaging in different neurodegenerative and neuroinflammatory conditions. Combined with his clinical expertise in different types of degenerative diseases and dementia, he has investigated the relationship between amyloid deposition, microglial activation, and glucose metabolism in different disorders, along with evaluating different transporters in the brain. His work in assessing microglial activation and amyloid load showed that both of these are increased in Alzheimer’s disease, and microglial activation correlates with cognition, while amyloid load does not correlate with cognition. He was an MRC clinical research fellow before he became a HEFCE clinical senior lecturer.
His work now focuses on neuroinflammation, and the interplay between inflammation and immunity in neurodegenerative and neuroinflammatory disease, and relating these with genetic information. He is also evaluating the methods of modulating inflammation and amyloid in Alzheimer’s disease, and the influence of cardiometabolic factors on the development of neurodegenerative diseases by means of clinical and pre-clinical studies.
He leads the Imperial College Memory Research Centre, and is the Chief Investigator of several imaging studies using PET and MRI, and heads multicentre studies evaluating novel treatment of Alzheimer's and other neurodegenerative diseases. He also runs a memory clinic at Imperial College Healthcare NHS Trust
IMPERIAL COLLEGE MEMORY RESEARCH CENTRE
At the Imperial College Memory Research Centre led by Dr Paul Edison, we focus our research on Alzheimer's disease, mild cognitive impairment, and causes of dementia and cognitive impairment. We evaluate neuroinflammation and neurodegeneration using PET, MRI, and CSF markers. We are now evaluating the relationship between
neuroinflammation, tau, glucose metabolism, and structural and functional changes measured by MRI. We heavily focus on basic and translational imaging using PET and MRI. We are evaluating novel therapeutic targets in dementia and cognitive impairment in Phase 1 and Phase 2/3 studies. We are evaluating novel microglial agents in mild cognitive impairment and Alzheimer's disease.
Additionally the unit uses state of the art techniques in PET and MRI in clinical evaluation of people with memory problems.
LIRAGLUTIDE IN TREATMENT OF ALZHEIMER'S DISEASE (ELAD)
There is a connection between type 2 diabetes and Alzheimer's disease; people with type 2 diabetes are much more likely to develop Alzheimer's disease than healthy people of the same age group.
The GLP-1 incretin analogue, liraglutide, is licensed and is being used safely in patients with type 2 diabetes mellitus. Experiments in AD transgenic mousemodels have shown that liraglutide crosses the blood-brain barrier, protects cognition, reduces amyloid plaqueformation, reduces inflammatory response, reduces levels of soluble amyloid oligomers, normalises synapticplasticity in the hippocampus, prevents progressive deterioration in glucose metabolism in the brain andincreases the proliferation of neuronal progenitor cells and the number of new neurons in the dentate gyrus. Liraglutide has demonstrated significant neuroprotective effect in preclinical studies and in a proof of concept PET study.
This is a 12-month, multicentre, randomised, double-blind, placebo-controlled Phase IIb study in patients with mild Alzheimer’s disease (AD) led and sponsored by Imperial College London. 206 patients will be randomised on a 1:1 ratio to receive liraglutide (1.8 mg daily by subcutaneous injection) or matching placebo from 20 centres around the UK and Ireland. This study aims to investigate the effect of liraglutide on glucose metabolism, MRI volume changes, amyloid load, tau formation and neuroinflammation using PET and MRI scans. Subjects will have brain scans at baseline and after 12 months of treatment. All scans are done at Imperial College Clinical Imaging Facility
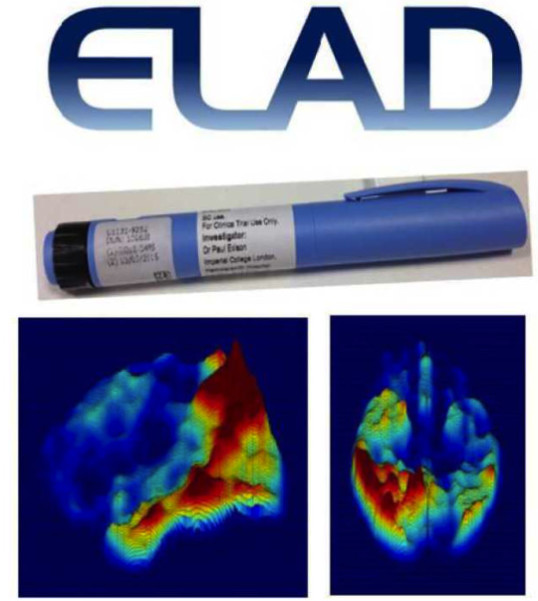
We are leading a multicentre clinical trial between 24 UK research sites, including King's College London, University of Cambridge, University of Southampton, University of Bristol, Birmingham University, University of Brighton, St Georges University of London evaluating the novel diabetic drug, liraglutide in the treatment of Alzheimer's disease (ELAD study). Liraglutide is currently licensed for diabetes, and has already passed through all the aspects of drug development.
To take part in any of our studies please contact us on
e-mail: memory@imperial.ac.uk or memory@imperial.nhs.uk
Tel: 020 8383 3704; 020 8383 1969
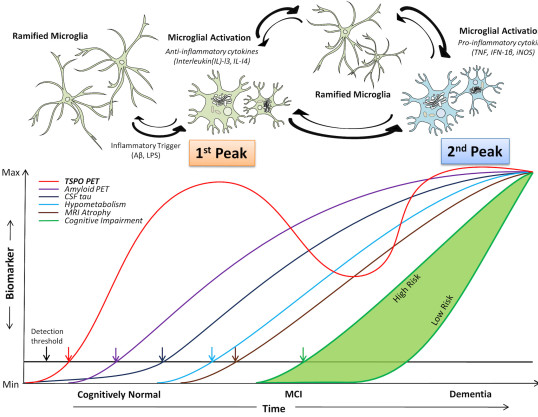
IMAGING AMYLOID AND NEUROINFLAMMATION IN SUBJECTS AT RISK FOR ALZHEIMER'S DISEASE
Neuroinflammation has been proposed to be a link between amyloid deposition, damage to neurons and formation of “tangles”. It has been shown that inflammation takes place with the deposition of amyloid in animals, but the relative timings have not been studied in humans. In this study, we are performing PET brain scans in 140 subjects using [11C]PBR28, a PET marker of neuroinflammation, while [18F]flutemetamol is used to evaluate deposition and [18F]AV1451 is a marker of tau deposition.
EVALUATION OF MICROGLIAL ACTIVATION IN ALZHEIMER’S DISEASE AND MILD COGNITIVE IMPAIRMENT SUBJECTS USING A NOVEL TSPO MARKER, GE180
[18F]GE180 is a novel PET imaging agent that has been developed for the assessment of neuroinflammation. TSPO levels in the normal central nervous system are very low, but increase dramatically in microglial cells in response to brain damage and inflammation. The TSPO tracer, [11C](R)PK11195, has been used for over 20 years to image brain inflammation. However, [11C]PK11195 has a very low specific to non-specific binding ratio and short radioactive half-life, making it a less than optimal imaging agent. In this study we are evaluating the novel tracer, [18F]GE180, in AD and other neurodegenerative diseases.
IMAGING AMYLOID AND NEUROINFLAMMATION IN SUBJECTS AT RISK FOR ALZHEIMER'S DISEASE
With PET we have previously shown that Alzheimer’s disease (AD) subjects have significantly increased accumulation of β-amyloid in the brain. In this research, we propose a proof-of-concept study to characterise the brain uptake of a novel astroglial activation imaging marker, [11C]BU99008, in a population of patients with mild to moderate AD. We will define the relationships between brain uptake of this marker, amyloid deposition (measured with [18F]florbetaben) and cerebral glucose metabolism (measured by uptake of [18F]FDG).
EVALUATING MICROGLIAL ACTIVATION AND REDUCED GLUCOSE METABOLISM IN LATER STAGES OF NEURODEGENARATIVE DISEASES
Genetic and post-mortem studies have highlighted an active role of neuroinflammation in the neuropathology of neurodegenerative diseases. The aim of this study is to assess whether neuroinflammation and related neuronal function in neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease.
AN IN VIVO EVALUATION OF ROLE OF AMYLOID IN DELIRIUM SECONDARY TO INFECTION
Patients who develop delirium have higher morbidity, mortality, institutionalisation, along with longer in-patient stay than non-delirious patients. Delirium is strongly associated with infection and other inflammatory processes in which pro-inflammatory cytokines play a role. Experimental evidence suggests that infection leads to increased production of pro-inflammatory cytokines and this could lead to delirium and accelerated neurodegeneration. In this study, we are evaluating the role of amyloid in delirium.
EVALUATION OF CASPASE ACTIVATION IN ALZHEIMER’S DISEASE USING [18F]ICMT11 PET/CT
The memory problems caused by Alzheimer’s disease are associated with high levels of abnormal proteins in the brain, one of which is β-amyloid. Build-up of amyloid proteins and high levels of the enzyme, caspase, are linked with neuronal damage and cell death in the brain. This study evaluates the novel PET radiotracer, [18F]ICMT11, which specifically highlights the parts of brain with high caspase levels. In this study we aim to look at caspase activation in the brain using the radiotracer [18F]ICMT11. We will also use MRI to compare with the PET scan
COMMERCIAL STUDIES
A 24-MONTH, MULTICENTER, RANDOMISED, DOUBLE-BLIND, PLACEBO-CONTROLLED, PARALLEL-GROUP, EFFICACY, SAFETY, TOLERABILITY, BIOMARKER, AND PHARMACOKINETIC STUDY OF AZD3293 IN EARLY ALZHEIMER’S DISEASE
Alzheimer’s disease (AD) is a progressive and fatal disorder of the nervous system, which results in amyloid plaque formation in specific regions of the brain, which is associated with inflammation and loss of neurones and synapses. AD is characterised by memory loss and progressively impairs activities of daily living.
Beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) is involved in the formation of amyloid plaques in AD. BACE1 is therefore considered to be a promising therapeutic target for slowing down or halting disease progression in AD. BACE1 splits amyloid precursor protein (APP) into a group of peptide fragments, which are the main component of the amyloid plaques. The study drug, AZD3293, is an inhibitor of BACE1 and has been shown to reduce peptide fragments in mice, rats, guinea pigs, dogs and humans. The study is being carried out to test whether AZD3293 modifies the clinical course of AD by delaying disease progression in patients diagnosed with early AD.
Team Members
Dr Paul Edison
Dr Gail Busza
Dr Daniella Femminella
Dr Valeria Calsolaro
Dr Fangda Leng
Harry Crook
Benjamin Fleming
Megan Young
James Breese
Frederick Page
Nicholas Livingston
Joseph Nowell
Dr Franco Mazzei Coria
Collaborators
Professor Mony De Leon, PhD – Professor of Neurology and Neuropsychiatry, New York University, USA
Professor Agneta Nordberg, MD, PhD – Professor/Senior Physician, Division of Translational Alzheimer Neurobiology, Karolinska Institute, Sweden
Professor Juha Rinne, MD, PhD – Professor of Neurotransmission, Turku PET Centre, Turku University Hospital and University of Turku, Finland
Professor Rik Vandenberghe, MD, PhD – Head of the Laboratory for Cognitive Neurology, Research Group Experimental Neurology, Leuven
Professor Pedro Rosa-Neto, McGill University, Canada
Professor Julie Williams, PhD – Professor of Genetics, MRC Centre for Neuropsychiatric Genetics & Genomics, Cardiff University
Professor Daniel Rueckert, PhD – Professor of Visual Information, Computational Science, Imperial College London
Professor Steve Williams, PhD – Professor and Head of Neuroimaging, Kings College London
Professor John Hardy, PhD, FMedSci, FRS – Professor of Neuroscience and Genetics, University College London
Professor Clive Ballard MBChB, MRCPsych – Professor of Age Related Disorders and Co-Director, Wolfson Centre for Age Related Disorders, Institute of Psychiatry/King’s College London
Professor Clive Holmes BSc, PhD, MRCPsych – Professor of Biological Psychiatry, University of Southampton
Prof. David J Brooks, MD, DSc, FRCP, FMedSci - Hartnett Professor of Neurology ,Department of Medicine, Imperial College, London
ELAD Study

There is currently no treatment that can prevent the disease and the only available treatments help with symptom control only. We are evaluating a possible future treatment for AD using an anti-diabetic drug that is already used for the treatment of diabetes.
There is a connection between type 2 diabetes and Alzheimer's disease; people with type 2 diabetes are much more likely to develop Alzheimer's disease than healthy people of the same age group. Recently, a drug used to treat type 2 diabetes, called liraglutide, has shown promise for the treatment of Alzheimer's disease. In laboratory studies it improves symptoms of Alzheimer's and reduces the amount of amyloid plaques in the brain, a hallmark of the disease.
This randomised, double blind, placebo controlled, multicentre clinical trial is led by Imperial College, and the participating centres include Kings College London, University College London, University of Oxford, University of Cambridge, University of Southampton, University of Bristol, Birmingham University, University of Brighton, St Georges University of London. Here we are evaluating novel diabetic drug Liraglutide intreatment of Alzheimer's disease (ELAD study) in 206 subjects. Lraglutide is currently licensed for diabetes, it has already passed through all the aspects of the drug development.
TEACHING

PHD
MSC
MRes
BSC
MBBS
-
I supervise - Post Doctoral Fellows
- PhD,
- MSc Neuroscience
- MRes Neuroscience
- BSc Neuroscience
- MBBS course
International Presentations

- The Differential Influence of Immune, Endocytotic and Lipid Metabolism Genes on Amyloid Deposition and Neurodegeneration in Alzheimer’s Disease: International conference on Alzheimer's disease. Chicago 2018
- Relationship between Astrocytes Activation Using [11C]BU99008 PET, Glucose Metabolism and Amyloid in Alzheimer’s Disease: A Dementia Platform UK Experimental Medicine Study. International conference on Alzheimer's disease. Chicago 2018
- Voxel-Level Interaction between NFT and Amyloid Influence/Predict the Decline Rate of the Cognition in Patients with Mild Cognitive Impairment: International Conference on Alzheimer's disease. Chicago 2018
- Microglial activation is associated with higher grey matter density and hippocampal volume in MCI subjects. Alzheimer’s Association International Conference; London, UK July 2017
- Dementia platform UK experimental medicine: human in vivo astroglial activation in early Alzheimer's disease. Alzheimer’s Association International Conference; London, UK July 2017
- Different modelling approaches for tau tracer 18F-AV1451 in mild cognitive impairment and Alzheimer's disease. Alzheimer’s Association International Conference; London, UK July 2017
- Strategies to develop parametric maps for TSPO PET Tracer [11C] - PBR28 in patients with mild cognitive impairment. Alzheimer’s Association International Conference; London, UK July 2017
- Regional kinetic modelling application for TSPO PET tracer [11C]PBR28. Alzheimer’s Association International Conference; London, UK July 2017
- Amyloid deposition, tau aggregation and microglial activation correlate with vascular burden in vivo in Alzheimer's disease. Alzheimer’s Association International Conference; London, UK July 2017
- Microglial activation in Alzheimer’s disease detected by novel third generation translocator protein tracer flutriciclamide ([18F]GE180). Alzheimer’s Association International Conference; London, UK July 2017
- Cardiometabolic risk predicts biomarkers progression in Alzheimer’s disease trajectory. . Alzheimer’s Association International Conference; London, UK July 2017
- Evaluation of caspase-3 activation in an Alzheimer’s disease population using [18F]ICMT-11 PET/CT. Alzheimer’s Association International Conference; London, UK July 2017
- Does microglial activation predate amyloid formation in Alzheimer’s disease trajectory? Alzheimer’s Association International Conference; Toronto, Canada July 2016
- Does microglial activation influence hippocampal volume and neuronal function in Alzheimer’s and Parkinson’s disease? Alzheimer’s Association International Conference: Washington DC July 2015
- Influence of microglial activation on neuronal function in Alzheimer's and Parkinson's disease dementia; American Academy of Neurology 2014
- Influence of neuroinflammation on neuronal function in neurodegenerative diseases: An 11C-(R)-PK11195 and 18F-FDG PET Study: Edison P, Fan Z, Yahyah A, Ahmed I, Chaudhury R K, Brooks DJ: American Academy of Neurology 2013
- Voxel-by-voxel correlation between amyloid load and microglial activation in Alzheimer and MCI subjects. Paul Edison, Fan Zhen, David J Brooks. Human Amyloid Imaging 2013
- Relationship between microglial activation, amyloid deposition and glucose metabolism in AD and PDD subjects. Edison, P and Brooks DJ. International Conference on Alzheimer’s Disease, Vancouver 2012
- Amyloid imaging in atypical dementia using [11C]PIB: International Conference on Alzheimer’s Disease: 2011 (Paris)
- Amyloid imaging in persenilin 1 mutation carriers with 11C-PiB-PET A. F. Ramlackhansingh, W. D. Knight, A. Okello, N. Ryan, F. Turkheimer, S. Rodriguez Martinez De Llano, P Edison, J. Douglas, N. C. Fox, M. N. Rossor, D. J. Brooks.
- Amyloid Imaging in Dementia: International Conference on Vascular Dementia, Budapest, Hungary, Nov 2007
- Amyloid load in Parkinson's disease dementia (PDD) and Lewy body dementia (LBD) measured with 11C-PIB PET: American Academy of Neurology, April 2007, Boston, MN, USA. (Scientific highlights)
- Amyloid imaging in AD, MCI, DLB, PDD, PD, and FTD using 11C-PIB PET:Human Amyloid Imaging, Boston, April, 2007
- Longitudinal Follow up of Alzheimer’s disease and mild cognitive Impairment in Alzheimer’s disease: International Conference on Alzheimer’s Disease, Madrid 2006
- Amyloid load, microglial activation and cognition: an 11C-PIB,11C-PK11195 and 18F-FDG PET study. American Geriatrics Society, Annual Meeting, May 2006, Chicago. (Best Paper Award)
- Amyloid deposition and metabolic dysfunction in Alzheimer’s disease and mild cognitive impairment: an11C-PIB and 18F-FDG PET study. 58th American Academy of Neurology, April 2006, San Diego, CA, USA (Scientific highlights)
- Microglial activation and amyloid deposition in Alzheimer’s disease: an 11C-PK11195 and 11C-PIB PET study. 58thAmerican Academy of Neurology, April 2006, San Diego, CA, USA. (Scientific highlights)
- Relationship between amyloid load, microglial activation and cognition: an 11C-PIB, 11C-PK11195 and 18F-FDG PET study:57th American Academy of Neurology, Miami April 2005 (Scientific highlights)
- Amyloid load in mild cognitive impairment compared to Alzheimer patients and normal subjects: An 11C-PIB PET study: Internationalconference on prevention of Alzheimer's and Dementia, Washington DC, June 2005
- Correlations between the distribution of microglial activation and amyloid plaque load in Alzheimer’s disease: an 11C-PK11195 and 11C-PIB PET study. International Conference on Prevention of Alzheimer's and Dementia, Washington DC, June 2005
- Cost effectiveness of BNP v/s ECHOin assessing cardiac failure in Primary Care Setting. European Union Conference of Geriatric Medicine at Florence, Italy April 2003
Collaborators
Dr Juha Rinne, University of Turku, Finland (Turku PET centre)
Dr Christopher Rowe, University of Melbourne (Centre for PET, Austin Health, Melbourne and Colin L Masters - Dept. Pathology)
Prof. Mony de Leon, New York University School of Medicine (Center for Brain Health)
Guest Lectures
New York University, USA: Amyloid Imaging in Dementia
International Conference on Vascular Dementia, 8-11 November 2007, Budapest
EUGMS (European Union Geriatric Medicine Society), Copenhagen 2008

