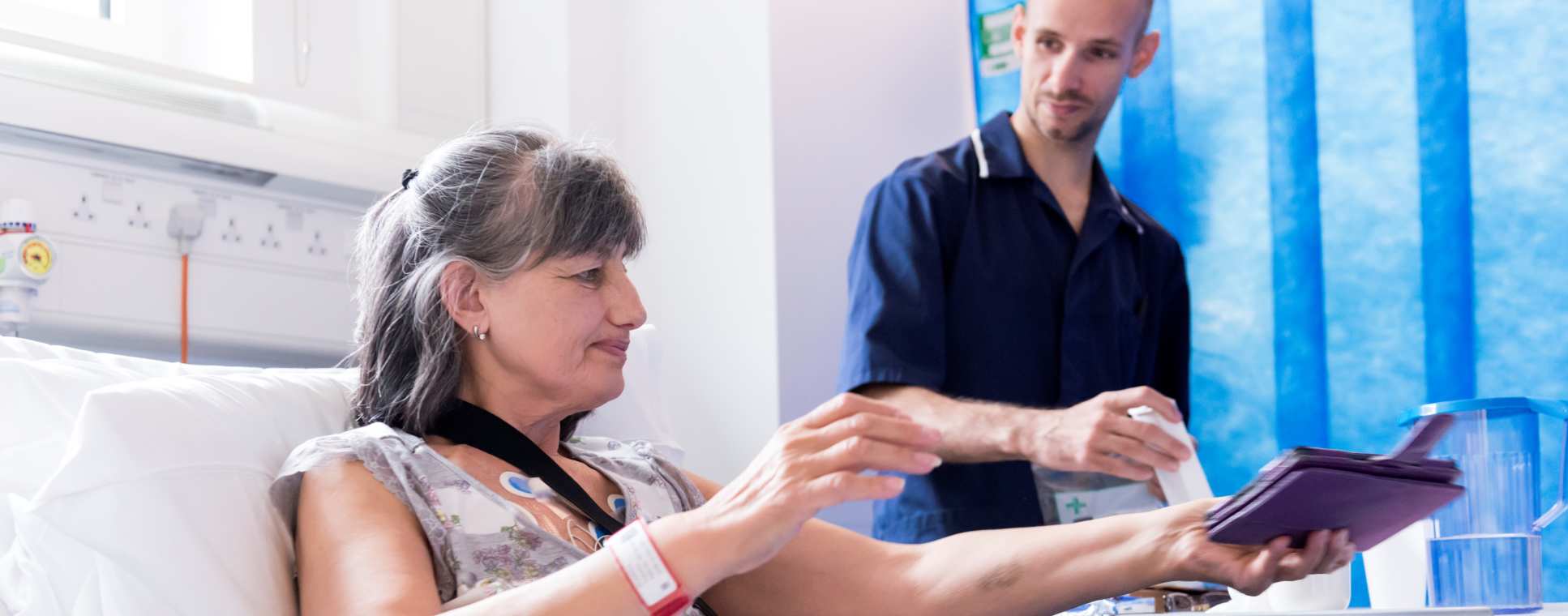
 Clinical trials are necessary to make sure new vaccines are safe and effective but are increasingly costly. Imperial is focused on more efficient ways to test vaccines while applying the knowledge gained from these trials to further understand and improve them.
Clinical trials are necessary to make sure new vaccines are safe and effective but are increasingly costly. Imperial is focused on more efficient ways to test vaccines while applying the knowledge gained from these trials to further understand and improve them.
- Vaccines need to be tested to make sure they are safe and effective. In every case, this must ultimately be done in people. Increasingly, the costs and risks of the large clinical trials required are limiting the speed at which we can develop effective vaccines and make them available.
- Imperial is highly focused on early-stage clinical trials in which new vaccines are tested for safety and ability to stimulate immune responses. Conducting these studies safely and efficiently helps to accelerate the development of new vaccines. Furthermore, understanding of how vaccines used in clinical trials are working allows us to go back and continually improve them, informing us about aspects of the pathogen; human immunology; and effectiveness of different delivery methods.
- Imperial is also at the forefront of experimental medicine strategies to drive forward vaccine development using controlled human infection models. Here, volunteers are deliberately infected with pathogens, including influenza, rhinovirus, respiratory syncytial virus (RSV) and malaria, allowing us to investigate human immunology in unprecedented detail.
Key members of this theme
Dr Sonya Abraham
/prod01/channel_3/media/migration/research-groups/Abraham,-Sonya--tojpeg_1510051847075_x4.jpg)
Dr Sonya Abraham
Senior Research Physician
Dr Andrew Blagborough
/prod01/channel_3/media/migration/research-groups/Blagborough,-Andrew--tojpeg_1510053346928_x4.jpg)
Dr Andrew Blagborough
Research Fellow
Dr Christopher Chiu
/prod01/channel_3/media/migration/research-groups/Chiu,-Christopher--tojpeg_1510045235715_x4.jpg)
Dr Christopher Chiu
Clinical Senior Lecturer
Dr Graham Cooke
/prod01/channel_3/media/migration/research-groups/Cooke,-Graham--tojpeg_1510052156777_x4.jpg)
Dr Graham Cooke
Reader in Infectious Diseases
Dr Victoria Cornelius
/prod01/channel_3/media/migration/research-groups/Cornelius,-Victoria--tojpeg_1510051551176_x4.jpg)
Dr Victoria Cornelius
Senior Lecturer in Clinical Trial Statistics
Professor Andrea Crisanti
/prod01/channel_3/media/migration/research-groups/Crisanti,-Andrea--tojpeg_1510051617588_x4.jpg)
Professor Andrea Crisanti
Professor of Molecular Parasitology
Dr Gavin Donaldson
/prod01/channel_3/media/migration/research-groups/Donaldson,-Gavin--tojpeg_1510052237696_x4.jpg)
Dr Gavin Donaldson
Reader in Airway Disease
Professor Christl Donnelly
/prod01/channel_3/media/migration/research-groups/Donnelly,-Christl--tojpeg_1510051684538_x4.jpg)
Professor Christl Donnelly
Professor of Statistical Epidemiology
Dr Ilaria Dorigatti
/prod01/channel_3/media/migration/research-groups/Dorigatti,-Ilaria--tojpeg_1510051770501_x4.jpg)
Dr Ilaria Dorigatti
Lecturer/Sir Henry Dale Fellow
Professor Stephen Durham
/prod01/channel_3/media/migration/research-groups/Durham,-Stephen--tojpeg_1510045323512_x4.jpg)
Professor Stephen Durham
Professor of Allergy and Respiratory Medicine
Professor Nicholas Grassly
/prod01/channel_3/media/migration/research-groups/Grassly,-Nicholas--tojpeg_1510053961405_x4.jpg)
Professor Nicholas Grassly
Professor of Infectious Disease & Vaccine Epidemiology
Dr Carolina Herrera
/prod01/channel_3/media/migration/research-groups/Carolina-Herrera--tojpeg_1571390949962_x4.jpg)
Dr Carolina Herrera
Senior Research Fellow
Professor Sebastian Johnston
/prod01/channel_3/media/migration/research-groups/Johnston,-Sebastian--tojpeg_1510045666112_x4.jpg)
Professor Sebastian Johnston
Asthma UK Clinical Chair
Professor Beate Kampmann
/prod01/channel_3/media/migration/research-groups/Kampmann,-Beate--tojpeg_1510045765512_x4.jpg)
Professor Beate Kampmann
Professor of Paediatric Infection and Immunity
Dr Kirsty Le Doare
/prod01/channel_3/media/migration/research-groups/Le-Doare,-Kirsty--tojpeg_1509959853844_x4.jpg)
Dr Kirsty Le Doare
Clinical Senior Lecturer in Paediatric Diseases
Dr Paul McKay
/prod01/channel_3/media/migration/research-groups/McKay,-Paul--tojpeg_1510045860646_x4.jpg)
Dr Paul McKay
Senior Research Fellow
Dr Simon Nadel
/prod01/channel_3/media/migration/research-groups/Nadel,-Simon--tojpeg_1510045983490_x4.jpg)
Dr Simon Nadel
Consultant in paediatric intensive care
Professor Peter Openshaw
/prod01/channel_3/media/migration/research-groups/Openshaw,-Peter--tojpeg_1510053266469_x4.jpg)
Professor Peter Openshaw
Clinical Consul for the Faculty of Medicine
Dr Katrina Pollock
/prod01/channel_3/media/migration/research-groups/Pollock,-Katrina--tojpeg_1510046751180_x4.jpg)
Dr Katrina Pollock
Clinical Lecturer
Professor Robin Shattock
/prod01/channel_3/media/migration/research-groups/Shattock,-Robin--tojpeg_1509963401104_x4.jpg)
Professor Robin Shattock
Chair in Mucosal Infection and Immunity
Dr Roger Tatoud
/prod01/channel_3/media/migration/research-groups/Tatoud,-Roger--tojpeg_1510052899260_x4.jpg)
Dr Roger Tatoud
Honorary Senior Research Officer
Professor Jonathan Weber
/prod01/channel_3/media/migration/research-groups/Weber,-Jonathan--tojpeg_1510052977836_x4.jpg)
Professor Jonathan Weber
Acting Dean of the Faculty of Medicine
Professor Jadwiga Wedzicha
/prod01/channel_3/media/migration/research-groups/Wedzicha,-Jadwiga--tojpeg_1510053070344_x4.jpg)
Professor Jadwiga Wedzicha
Clinical Chair in Respiratory Medicine
Professor Robert Wilkinson
/prod01/channel_3/media/migration/research-groups/Wilkinson,-Robert--tojpeg_1510046938799_x4.jpg)
Professor Robert Wilkinson
Professor in Infectious Diseases
General enquiries
Clinical Senior Lecturer
Dr Christopher Chiu
vaccine.network@imperial.ac.uk
+44 (0)20 8383 2301
