H1 Hip Resurfacing Arthroplasty Clinical Investigation
REC REF: 17/EE/0330
This is a 10-year multi-centre, prospective, non-randomised, observational study to evaluate the clinical outcome of The H1 Hip Resurfacing Arthroplasty (The H1 Implant, Figure 1). The H1 Implant was designed and is manufactured by Embody Orthopaedic, the sponsor of the clinical investigation. The patients are fully recruited, so while follow up will continue until 2032, no new patients are being admitted.
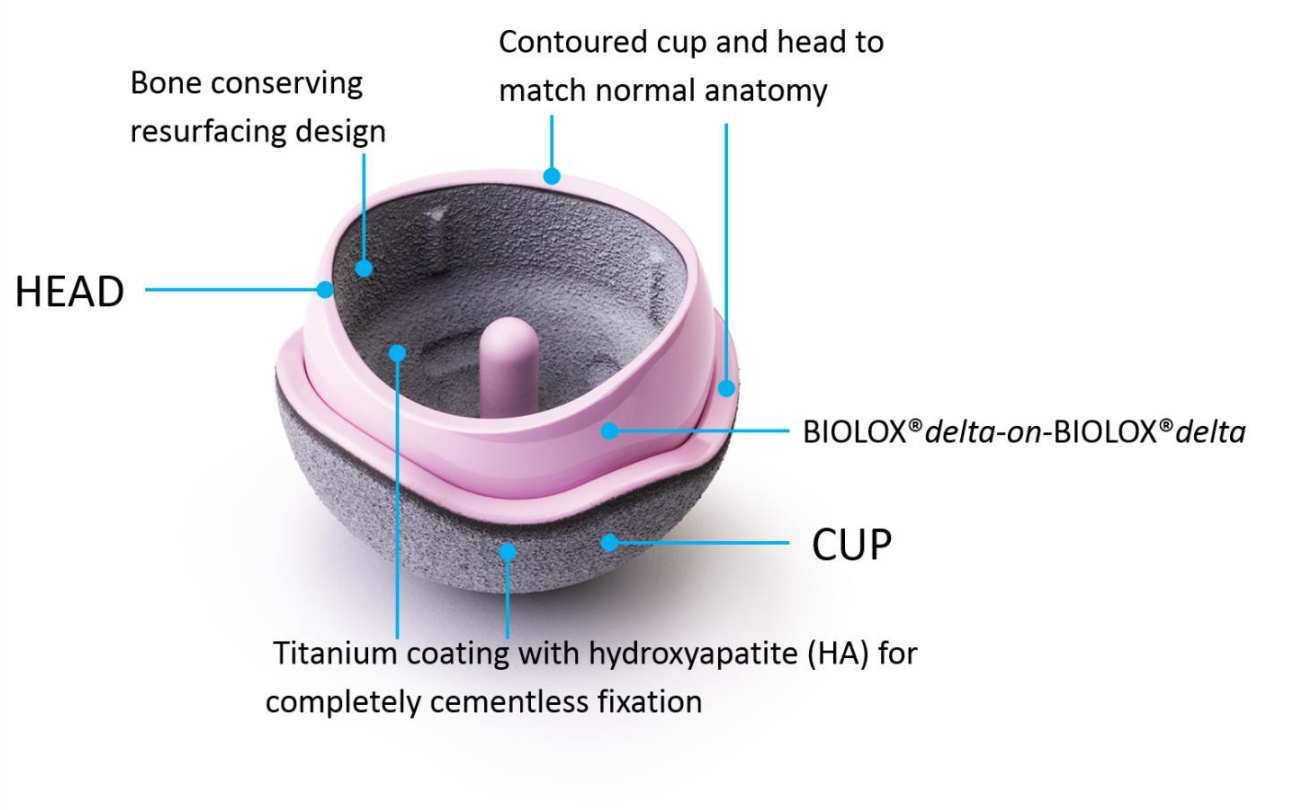
The H1 Implant
STUDY OBJECTIVE
The primary objective of this study is to confirm the safety and efficacy of the H1 Implant by demonstrating non-inferiority compared to the Birmingham Hip Resurfacing (where revision of the device is deemed a failure). The secondary objective is to demonstrate the superiority of the H1 Implant with its metal-free articulation compared to metal-on-metal hip resurfacing in the absence of metal ion release.
OVERVIEW
Modern Hip Resurfacing Arthroplasty (HRA) was introduced in the 1990s to address inferior implant survivorship and unsatisfactory clinical results with total hip arthroplasty in young and active patients.
Metal-on-Metal (MoM) HRAs have been shown to be safe and effective in younger patients over the longer term. However, patients (especially females) with poorly positioned HRAs, poorly designed HRA implants, and smaller sizes have reported progressive pain leading to early revision. The pain is commonly caused by adverse local tissue reactions to metal ion particles that are generated by excessive wear.
The H1 Implant is a ceramic-on-ceramic, cementless HRA. By exchanging the metal material of the bearing with BIOLOX®delta ceramic (CeramTec), a better wearing and more inert material, the positive clinical performance aspects of MoM hip resurfacings are retained, while the main cause of early revision is removed. Thus, the H1 Implant could be used for wider indications than the currently restricted group of large men that applies to MoM HRAs. Patients with smaller head sizes, females, and patients with metal sensitivity may all be H1 Implant candidates.