New materials could make greener fast-charging batteries
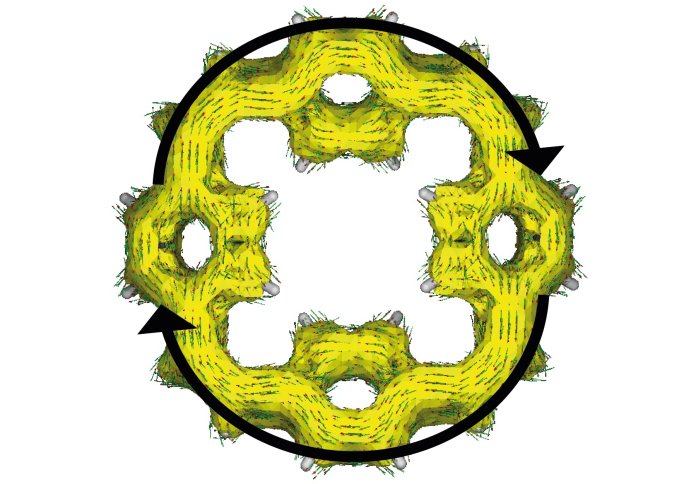
Distribution of the electrons in the macrocyclic molecules of the electrode after charging the battery
Researchers have created a fast-charging battery prototype that uses sodium instead of lithium, potentially leading to more sustainable batteries.
The prototype is one of the first to successfully use sodium in an organic battery that can be quickly charged and discharged hundreds of times without losing any capacity.
Many people have concerns about the widespread use of lithium-ion batteries and their environmental implications, which these new sodium-ion batteries could help address. Dr Florian Glöcklhofer
Although the total capacity (power storage) is currently lower than common commercial lithium-ion batteries, the team are working on improvements and say the new prototype is a big step forward.
Because sodium is far more abundant than lithium, it is easier to extract, so requires less mining and is more environmentally friendly. The prototype also uses materials that could replace heavy metals, which are also mining-intensive.
The details of the new prototype are published today in Angewandte Chemie.
Lead researcher Dr Florian Glöcklhofer, from the Department of Chemistry and Centre for Processable Electronics at Imperial, said: “Many people have concerns about the widespread use of lithium-ion batteries and their environmental implications, which these new sodium-ion batteries could help address.
“Many people have also experienced the problem of having to charge their devices more often as they get older and the batteries lose capacity. Our battery prototype did not lose any capacity when tested over 500 charge-discharge cycles, meaning it should not face this problem if developed into a commercial product.”
Room for sodium ions
Batteries rely on electrode materials to store and release electrons and positively charged ions for charging and discharging power. Most batteries today, such as those in a laptop or mobile phone, use lithium ions.
Sodium ions are larger than lithium ions, and it has been difficult for researchers to find electrode materials that can store sodium ions. For the new prototype, researchers used organic materials known as ‘macrocyclic molecules’ for the electrodes. These are made up of only hydrogen and carbon atoms, and form ring structures with central holes.

Like a pile of pineapple rings, these macrocyclic molecules will always have some space in their centre, no matter how densely they are stacked. This space is sufficient to allow sodium ions to be stored without distorting the material.
Stable distribution of electrons
In addition, the ring structure of the macrocyclic molecules allows stored electrons to be distributed around the ring. As they are more spread out than when stored in some other electrode materials, the electrons are less likely to react with elements of the battery. This means the battery remains stable and does not lose any capacity each time it is charged and discharged.
The structure of the macrocyclic molecules also means two electrons are always taken up together, increasing the performance and making charging fast.
While the battery prototype can be charged and discharged quickly, and is stable over many cycles of charge and discharge, the overall power capacity is lower than commercial lithium-ion batteries. The team are working to improve the electrode materials and raise their capacity, potentially bringing sodium-ion batteries to the market in the future.
The project received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 796024.
-
‘Switching between local and global aromaticity in a conjugated macrocycle enables high-performance organic sodium-ion battery anodes’ by Simon Eder, Dong-Joo Yoo, Wojciech Nogala, Matthias Pletzer, Alejandro Santana Bonilla, Andrew J.P. White, Kim E. Jelfs, Martin Heeney, Jang Wook Choi and Florian Glöcklhofer is published in Angewandte Chemie.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Hayley Dunning
Communications Division