Summary
Aran Singanayagam is an MRC Clinician Scientist, Group Leader at the Centre for Molecular Bacteriology and Infection (CMBI) at Imperial College and Consultant in Respiratory Medicine at the Royal Brompton and Harefield NHS trust.
Aran qualified from the University of Edinburgh medical school in 2005 achieving distinction in his final examinations. He intercalated with a BSc. in Physiology and was awarded First Class Honours. In 2009, he was appointed to the post of NIHR Academic Clinical Fellow in Respiratory Medicine at Imperial College. A Wellcome Trust Clinical Research Training Fellowship enabled him to undertake a PhD. using experimental models to understand how the commonly used inhaled therapy fluticasone propionate can impair immune responses to rhinovirus infection. He identified type I interferon as a central regulator of antibacterial immunity and mucus production during virus-induced exacerbations of COPD. This work led to a first author publication in Nature Communications (accessible here).
Aran then took up an NIHR Academic Clinical Lecturer post to develop a niche studying mechanisms of dysregulated host-defence in chronic lung disease. He built upon his PhD. work by demonstrating that inhaled corticosteroids can perturb the airway microbiota and impair bacterial control through suppression of the anti-microbial peptide cathelicidin, an effect that occurs mechanistically through augmentation of the protease cathepsin D, a negative regulator of cathelicidin. This work has been published in Science Translational Medicine (accessible here).
Subsequently, Aran was awarded a Wellcome Trust ISSF Springboard Fellowship followed by a prestigious MRC Clinician Scientist Fellowship which enabled him to establish his research group studying the role of the respiratory tract microbiota in innate immunity.
Aran has received prestigious early career investigator awards from the British Thoracic Society (BTS), the European Respiratory Society (ERS) and the Worshipful Society of Apothecaries. He was a member of the BTS Science and Research Committee (2015-2018) and the ERS Long Range Planning Committee for Respiratory Infection (2015-2018). He sits on the Editorial Board of American Journal of Respiratory and Critical Care Medicine and European Respiratory Journal and has published over 100 papers to date with a H index of 45.
RESEARCH INTERESTS
Aran's research program focusses on how pulmonary host-defence is dysregulated in the context of inflammatory airway diseases. Further details of can be found on the lab website here. Major research themes currently include:
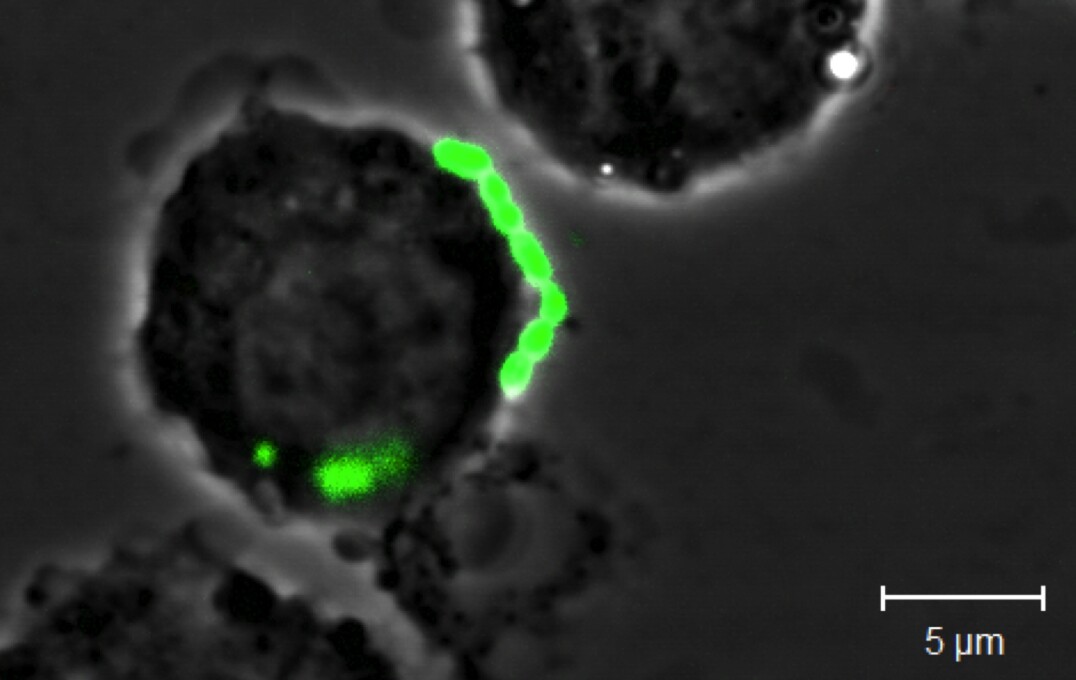
Understanding the role played by the respiratory tract microbiota in innate anti-microbial immunity: We are using cutting-edge immunological and molecular microbiological techniques in human and animal disease models to gain functional understanding of roles played by the respiratory tract microbiota. We are focussing on how commensals within the microbiota regulate immune homeostasis in health and how perturbations that occur in chronic lung diseases lead to immune dysregulation and impaired protection against pathogens. Our work has been discussed in a webcast (accessible here).
Investigating the role of respiratory tract mucins in mucosal host-defence: With funding from the British Lung Foundation and British Medical Association, we are examining the role of respiratory tract mucins in innate immune responses to viral and bacterial pathogens in asthma and COPD. Our research was featured on World COPD Day 2017 (see details here) and is published in Journal of Clinical Investigation (accessible here).
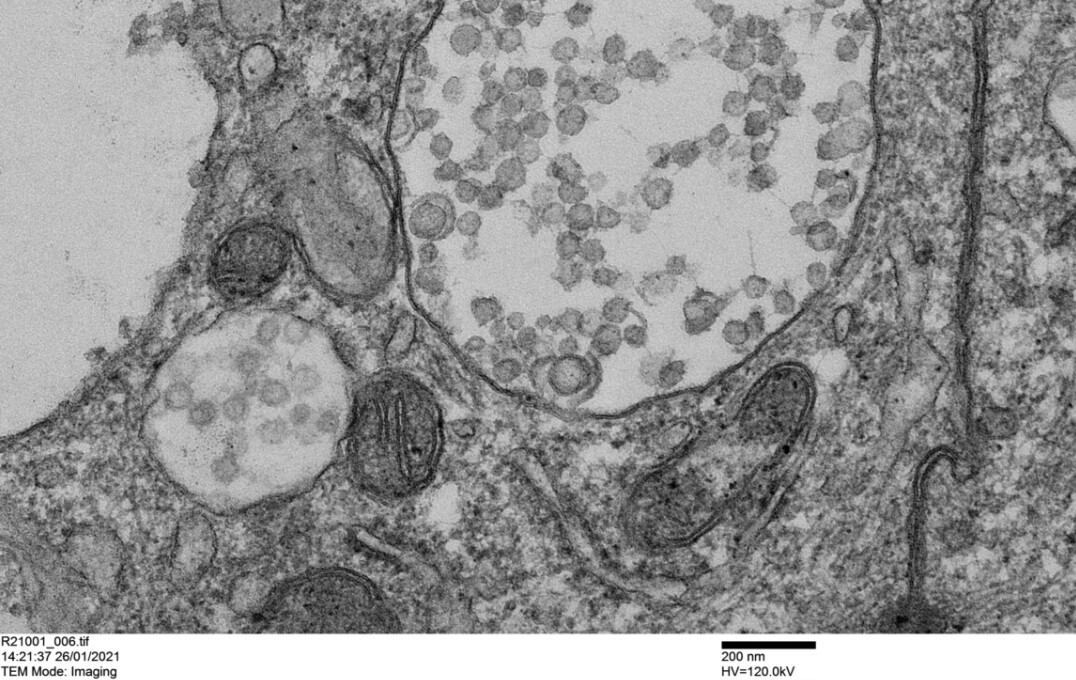
Mechanisms of susceptibility to acute COVID-19 and its sequelae : We are combining ex vivo approaches with analyses of in vivo samples from patients to determine what drives altered susceptibility to severe COVID-19 and its sequelae with the aim of identifying novel druggable targets. Our work elucidating mechanisms driving post COVID-19 lung sequelae was featured in a news release (see details here) and has been published in Science Translational Medicine (accessible here). Our work on effects of inhaled therapies upon SARS-CoV-2 entry receptors was also featured in a news release (see details here) and has been published in Journal of Allergy and Clinical Immunology. (accessible here).

GROUP MEMBERS
Current:
- Millie Jackson (Research Technician)
- Orestis Katsoulis (Research Technician)
- Oliver Pitts (Research Assistant)
- Nikhil Patel (Clinical PhD Research Fellow)
- Rose Nixon (Clinical MD Research Fellow)
Alumni:
- Tasnim Faiez (Research Technician)
- Faisal Kamal (Clinical PhD Research Fellow)
- Erica Jong (MSc. Immunology project student)
- Matas Skiotys (MRes. Bacterial Pathogenesis and Infection project student)
- Fatima Faizi (MSc. Immunology project student)
- Ivan Chan (MRes. BPI project student)
- Wangmingyu Xia (MRes. Microbiome project student)
- Hamsa Narasimhan (MSc. Immunology project student)
Please get in touch to discuss clinical or non-clinical PhD. and postdoctoral research opportunities.
Selected Publications
Journal Articles
Almond M, Farne HA, Jackson MM, et al., 2023, Obesity dysregulates the pulmonary antiviral immune response., Nat Commun, Vol:14
George PM, Reed A, Desai SR, et al., 2022, A persistent neutrophil-associated immune signature characterizes post-COVID-19 pulmonary sequelae., Science Translational Medicine, Vol:14, ISSN:1946-6234, Pages:1-16
Singanayagam A, Footitt J, Marczynski M, et al., 2022, Airway mucins promote immunopathology in virus-exacerbated chronic obstructive pulmonary disease., Journal of Clinical Investigation, Vol:132, ISSN:0021-9738, Pages:1-16
Kamal F, Kumar S, Edwards MR, et al., 2021, Virus-induced volatile organic compounds are detectable in exhaled breath during pulmonary infection., American Journal of Respiratory and Critical Care Medicine, Vol:204, ISSN:1073-449X, Pages:1075-1085
Finney L, Glanville N, Farne H, et al., 2021, Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon, Journal of Allergy and Clinical Immunology, Vol:147, ISSN:0091-6749, Pages:510-519.e5
Kamal F, Glanville N, Xia W, et al., 2020, Beclomethasone has lesser suppressive effects on inflammation and anti-bacterial immunity than Fluticasone or Budesonide in experimental infection models., Chest, Vol:158, ISSN:0012-3692, Pages:947-951
Singanayagam A, Glanville N, Cuthbertson L, et al., 2019, Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease, Science Translational Medicine, Vol:11, ISSN:1946-6234, Pages:1-13
Singanayagam A, Glanville N, Girkin J, et al., 2018, Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations, Nature Communications, Vol:9, ISSN:2041-1723, Pages:1-16