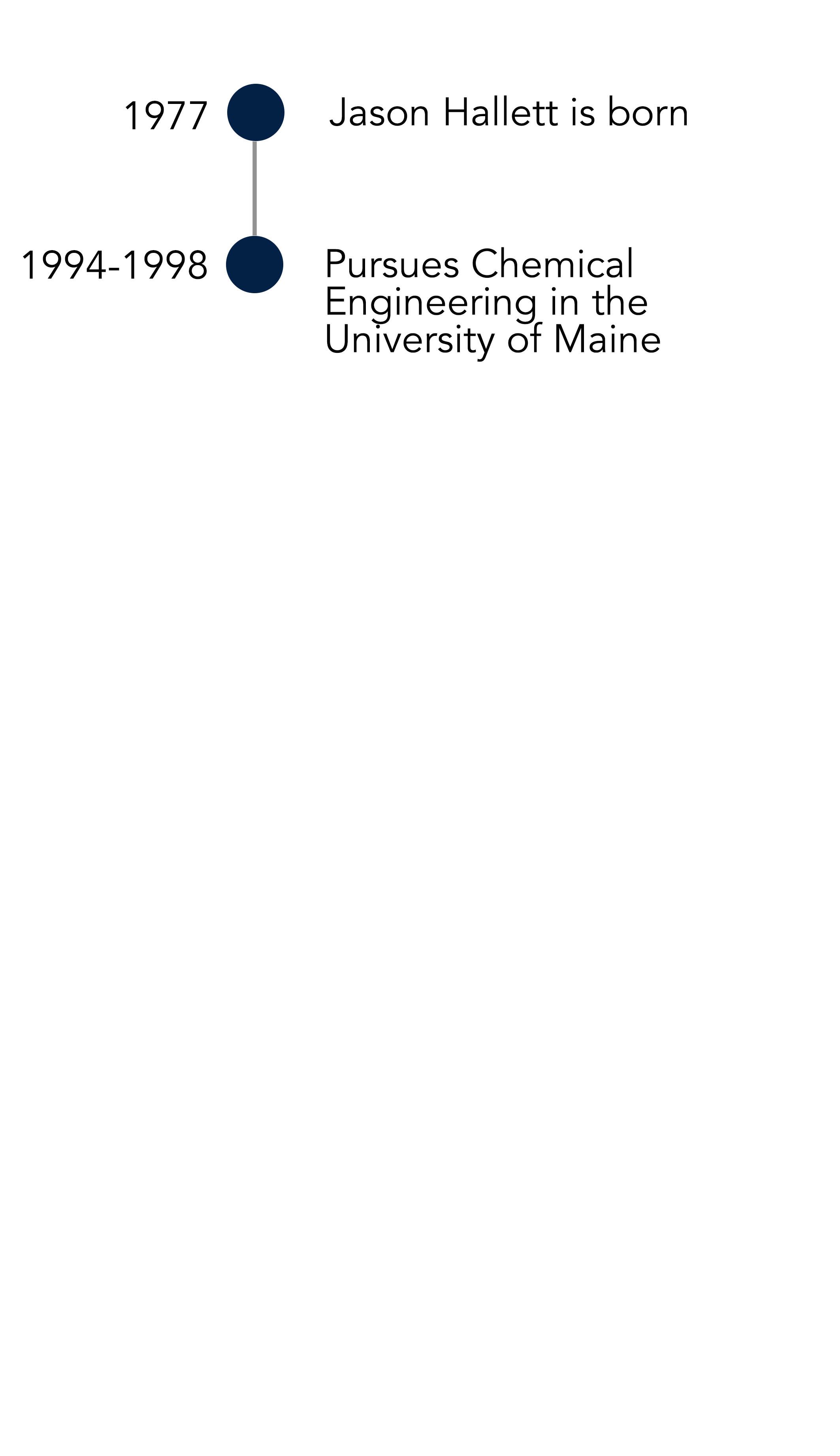"I decided I was going to use engineering to solve chemistry problems"
How chemical engineer Jason Hallett went from working at a paper mill to revolutionising the industry

“I don’t recommend to any of my students or postgraduates take the path that I’ve taken,” Professor Jason Hallett says to me.
We are speaking over a video call with Jason calling from the Imperial Chemical Engineering office. I can hear loud construction noises coming from Jason’s background, so our discussions about how he came to be the co-founder of six spin-out companies are overlaid with sounds of drilling and hammering.
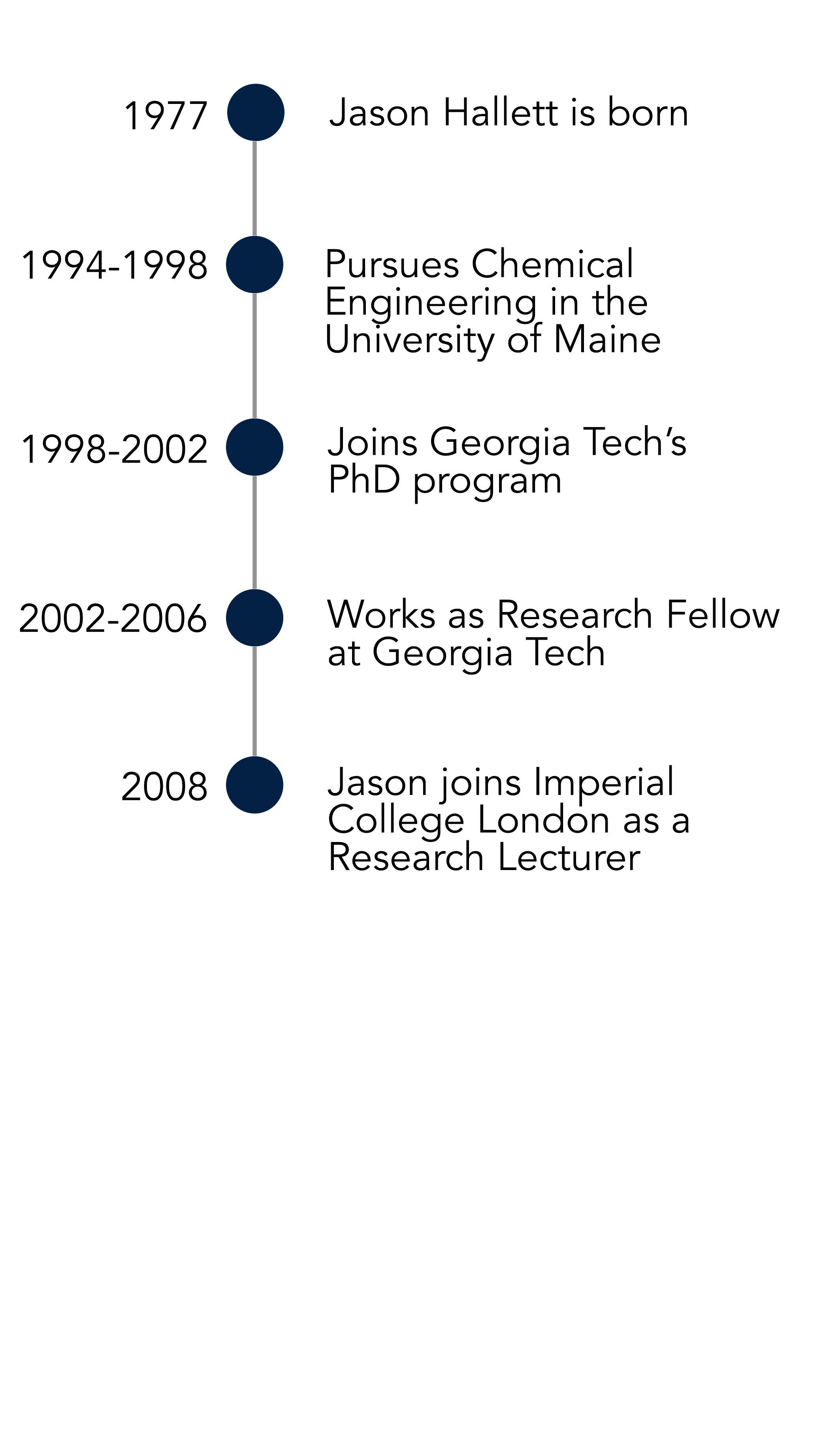
He explains to me that he’s faced significant pushback to his approach to work, and he can in no good conscience advise that anyone practice what he refers to as the ‘engineer-ification of chemistry’. However, it’s unclear why Jason seems reticent to let his student follow in his footsteps. He has been involved in founding not one, but six spin-out companies since he started as a research lecturer in Imperial College London in 2008 – surely more than enough to inspire others to follow suit?
“There was this sort of attitude that I faced early on, that, ‘This is just not how things are done’,” Jason says, “I got told that by so many expert people but if it works, who cares how I did it, right?” Jason says he was warned about being a one-trick pony in one of his early job interviews – it’s a similar cautionary tale to the one his PhD supervisor issued him before: if you’re holding a hammer, everything begins to look like a nail.
[You’re] not supposed to try and decipher the answer upfront when you do science. It’s supposed to be about discovery.
Hallett’s hammer is the process of working backwards from a solution. When faced with a practical problem, he says we often already have multiple solutions. However, the reason we don’t adopt them is because they have a set of problems of their own: too expensive, too unstable, too many unwanted by-products. Rather than starting from zero and creating a completely new solution, Jason asks why not just work backwards and slowly fix the solution we already have? “The conventional wisdom is that this is the worst thing you can possibly try to do, because you’re not supposed to try and decipher the answer upfront when you do science,” Jason says, “It’s supposed to be about discovery.”
With the great success of his ‘hammer’ approach, Jason tells me he now fortunately finds chemistry tolerable, but his true fixation in childhood was mathematics.
His hometown is a small town in rural Maine called Passadumkeag, surrounded by natural forest. In the winters, Passadumkeag becomes buried in snow and temperatures can drop to as low as -30°C.

The frigid temperatures may have contributed to his future penchant for wearing Hawaiian shirts at every given opportunity
They were out of math courses. Nobody around knew anything better and I had to wait around until I went to university to learn anything more.
Around 300 people live in Passadumkeag, all mainly working in the town’s forestry industry, with one road leading in and out. The nearest small city was 300 kilometres away and the nearest major city was almost 1000 kilometres away, which meant that Jason spent much of his childhood working through mathematics textbooks he was given. “I learned calculus, I was probably around twelve, but then that was it,” he says. “They were out of math courses. Nobody around knew anything better and I had to wait around until I went to university to learn anything more.”
He would go on to be the Maine State Math Champion, and then went on to win the regional competition for New England. Despite several victories at the state and regional levels, as well as a prestigious story in the local newspaper where they named Jason the ‘Athlete of the Week’, there were not many other opportunities for young upstarts. The only real industry, he says, was the paper mill located the next district over in Lincoln.
Determined to go to a bigger city or town for university, Jason only applied to two schools. “I was positive I was going to go to MIT, so I applied for electrical engineering, but just in case I didn’t get the scholarship, I applied to the local state university,” he says. Unlike MIT, the University of Maine was only really known for their chemical engineering department, which was heavily linked to the pulp and paper industry.
When the grand plan for an MIT scholarship fell through, Jason ended up taking a scholarship from the Pulp and Paper Institute to study chemical engineering at the University of Maine. An uninspiring internship at a Rumford paper mill later, Jason would abandon any contact with the industry until almost a decade later when he’d revolutionise it.
We didn’t always behave ourselves; we would stay in the laboratory until one o’clock in the morning, sometimes we go out for dinner and then go back for work.
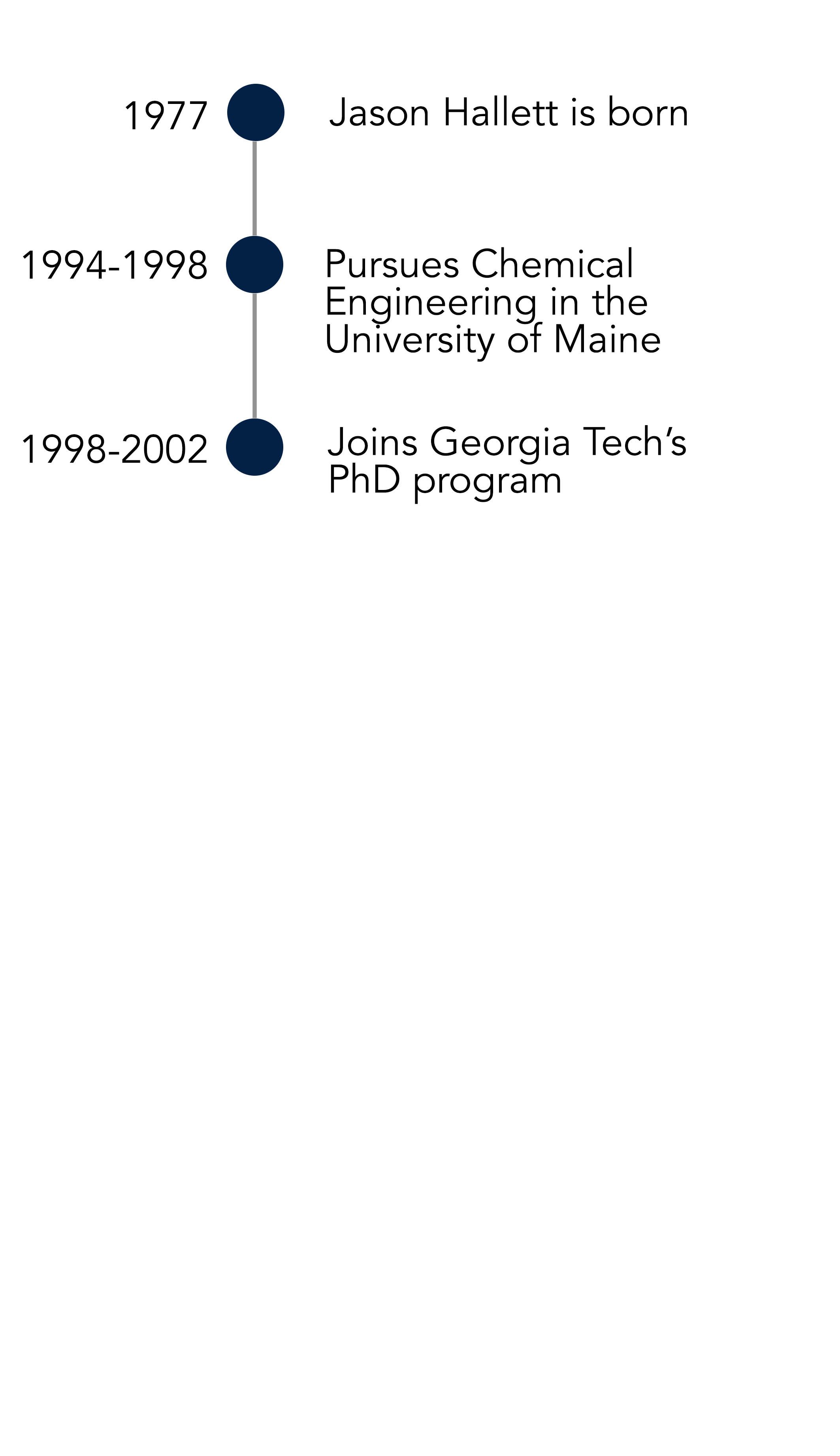
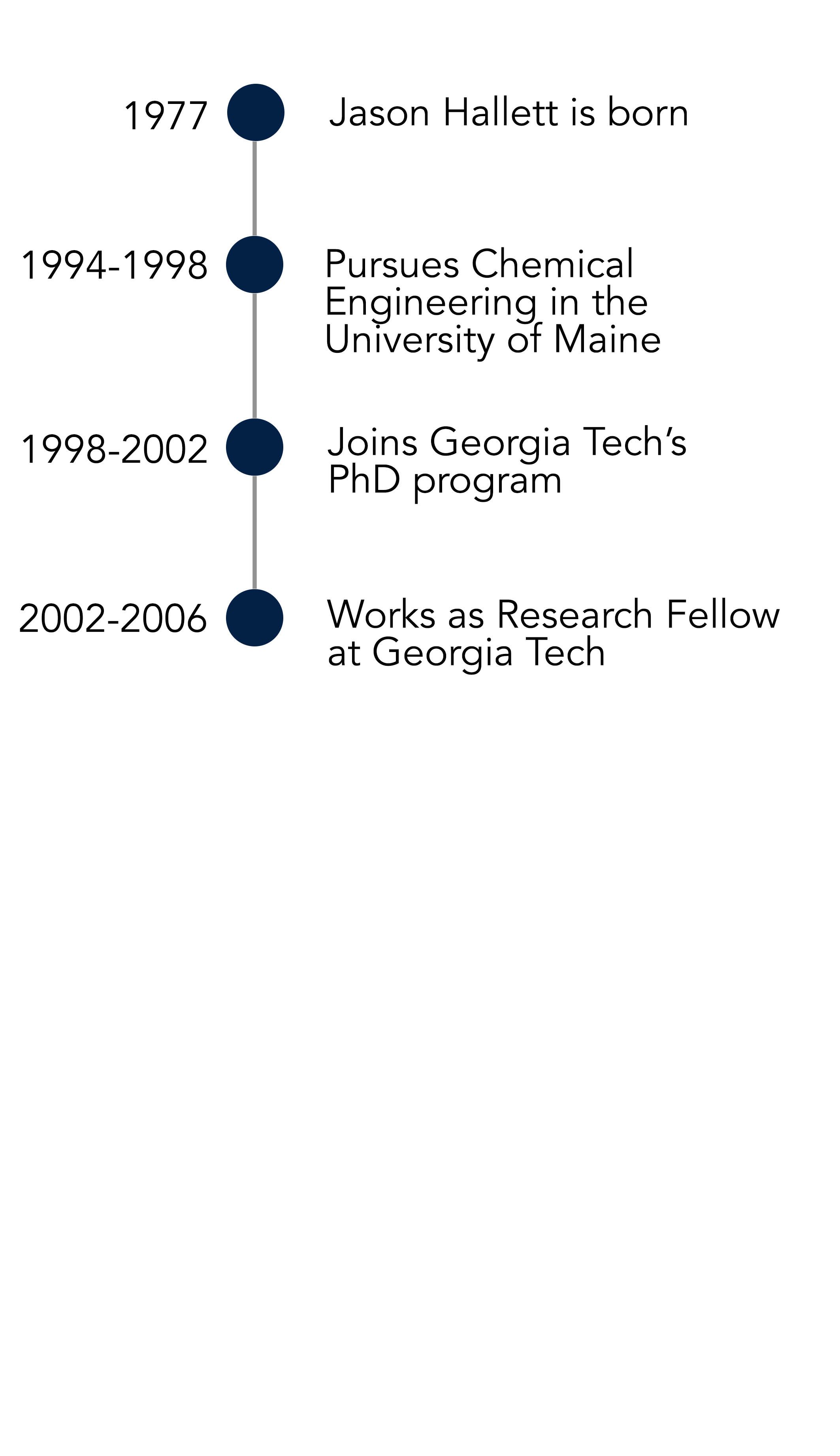
For the meantime, he would receive his postdoctoral degree from Georgia Institute of Technology (otherwise known as ‘Georgia Tech’). “When I started my PhD, I didn’t really know what to expect – the first research paper I read was when I was preparing for my PhD-qualifying exam; I had never read one before that,” he says. “It was just such a different environment: intellectually stimulating. We didn’t always behave ourselves; we would stay in the laboratory until one o’clock in the morning, sometimes we go out for dinner and then go back for work.”
Working in a laboratory allowed Jason to jump from topic to topic, in all publishing 12 papers by the end of his PhD – staying in the broad area of multi-phase, high-temperature and high-pressure reactions. His supervisor was Charlie Liotta and Chuck Eckert – an established chemist and chemical engineer (respectively) who offered Jason valuable life advice: “Chuck told me that the problem I had to avoid was the temptation to be a bad chemist. That’s always stuck with me. I never listened to it though!” he says.
“Traditionally, chemists try to use chemistry to solve engineering problems, which doesn’t work very well. I decided I was going to use engineering to solve chemistry problems.”
The biggest problem that Jason has managed to solve echoes back to his roots: biorefining in wood pulping – the biggest commercial application of which is to make paper. Another goal in wood pulping, however, was to convert the sugars in cellulose fibres – the long, strong molecules that give plants their structure – into ethanol. Usually, this process organically happens when naturally-occurring microbes eat the sugars trapped in cellulose and convert them into alcohol. To protect themselves from hungry microbes, trees also have a polymer called lignin that is toxic.
Wood pulping (otherwise known as the kraft process) converts wood into pulp. It involves mechanically and chemically treating wood chips to break the bonds that link lignin, hemicellulose and cellulose. These three polymers make up what we know as wood. The kraft process is the main way the world creates paper, but it produces huge amounts of liquid and air pollution.
A large portion of the modern biorefining industry seeks ways to separate lignin from biomass. “It’s the fifth biggest source of industrial pollution, which considering the big three – concrete, steel and petroleum refining – is not a great place to be,” Jason says.
Chemists have been attempting to develop techniques to extract lignin from biomass that are non-polluting. Jason’s own research group investigated ionic liquids, which are solvents that don’t evaporate at room temperature and therefore cannot cause air pollution. Since 2007, they experimented with dissolving and isolating cellulose in biomass using ionic liquids when one of his postdoctorate researchers, Agnieszka Brandt-Talbot, observed some strange behaviour in the laboratory.
“Agi discovered in the lab that one ionic liquid was dissolving half of the biomass but not the other half. She brought me the result and asked what the hell was going on,” Jason says, “and I said that looks exactly like organosolv pulp.”
Organosolv, in industrial paper-making, is a technique where an organic solvent is used to dissolve lignin and hemicellulose. Commercially, organosolv pulping has been expensive – keeping its use rather niche; Jason himself had worked on it during his time at Georgia Tech. What had been happening was that Agnieszka’s ionic liquid was failing to dissolve the cellulose, but was succeeding at dissolving everything else.
Here was the simple answer that would flip industrial problems on their heads. Instead of trying to find a high-cost ionic liquid that could dissolve cellulose, Jason would help develop a low-cost ionic liquid that would dissolve but the lignin. In the end, the results are the same: you isolate the cellulose. Hammer and nail. Jason knew the significance: “When you take the lignin out of wood, then you can turn the cellulose into ethanol.”
I’d love to say that I was a visionary, that I was ahead of the trend on that, but it really wasn’t me – I was kind of dragged along.
That test tube experiment is now being used by a chemical plant that will process 100 tonnes of wood a year. Jason has spent the past four years as one of the founders of Lixea. Most of that time was spent figuring out how to make the ionic liquid extremely cheaply and unlocking its commercial potential.
“I’d love to say that I was a visionary, that I was ahead of the trend on that, but it really wasn’t me – I was kind of dragged along,” Jason says. He states he was only ever interested in making an impact, for the solutions they developed to be used and to avoid the academic tendency to do what he calls “navel gazing”.
Agnieszka, now a Research Fellow, was the one who wanted to form a spin-out company and Jason had capitulated in order to lure her back from her job offer in industry.
One spin-out company eventually led to many, many others. Developing Lixea’s ionic liquids for commercial use meant Jason had to study the solvent’s corrosion behaviour. Any business that used ionic liquids at appreciable scales needed to fully understand how the solvent would behave in an industrial reactor that also holds high-temperature, water, salt and acid.
After receiving funding from the Institute of Science and Engineering to study the corrosive behaviour, one of Jason’s students had discovered that their experimental samples were forming strange structures: “This is always the story: one of the solvents always acts weird.”
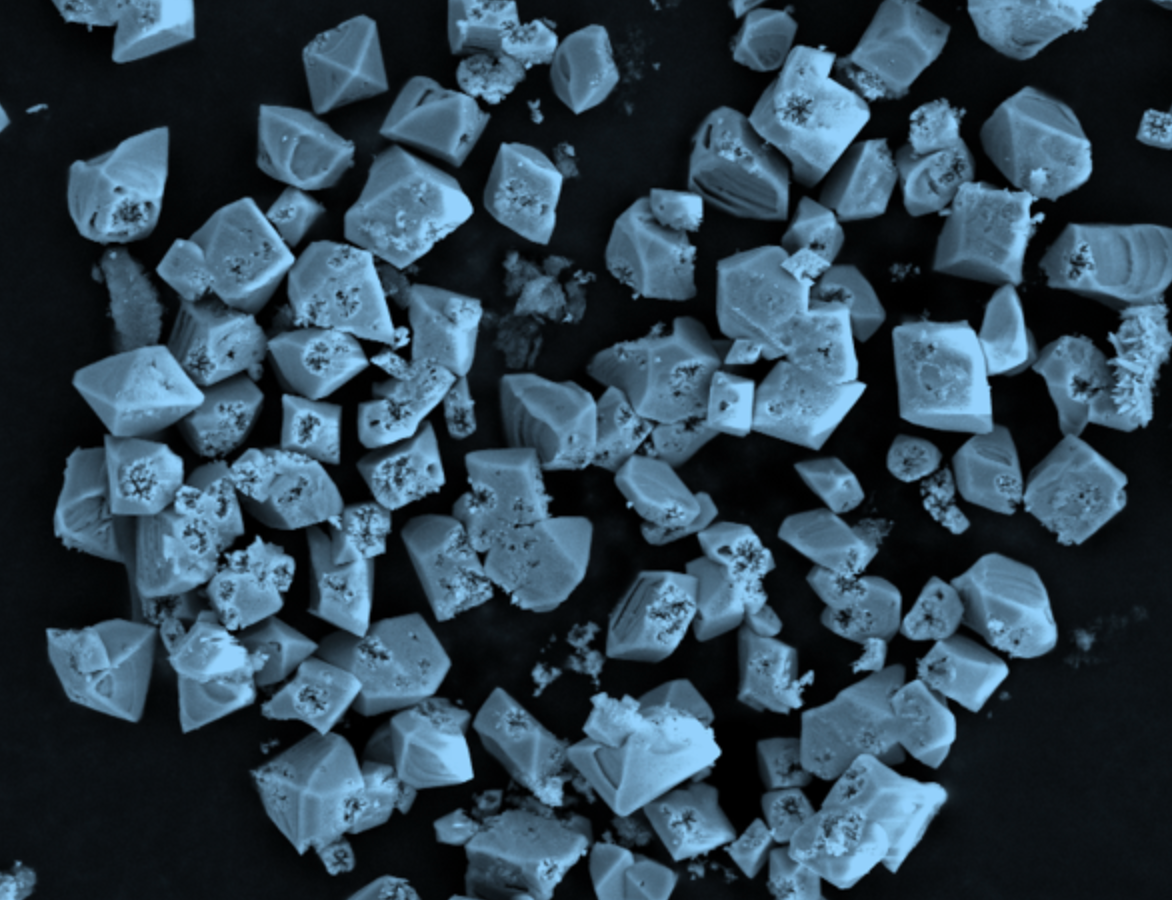
The student, Francisco Malaret, was studying electron microscope images of the corrosion and found that they were forming octahedral structures. “We did some literature searching and it turns out, not only were the octahedral structures unusual, but people actually wanted them – people were going to great lengths to grow these crystals and here they are, in our case, just happening,” Jason says.

Octahedral zinc oxide nanocrystals that grew on the surface of zinc (or brass)
Octahedral zinc oxide nanocrystals that grew on the surface of zinc (or brass)
Octahedral zinc hydroxide particles, coloured blue
Octahedral zinc hydroxide particles, coloured blue
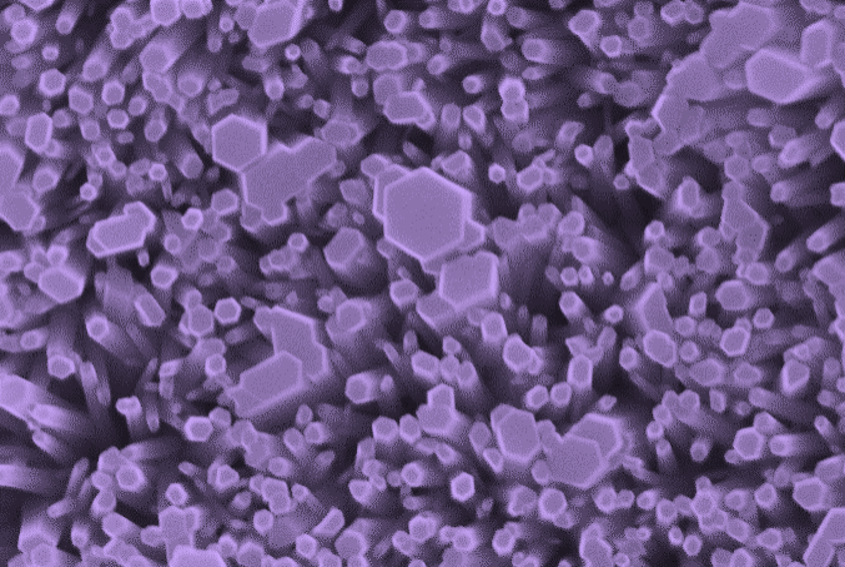
Octahedral zinc oxide nanocrystals, coloured purple
Octahedral zinc oxide nanocrystals, coloured purple
People were going to great lengths to grow these crystals and here they are, in our case, just happening.
Ionic liquids could not only dissolve lignin out of wood, but Jason’s research group also found it could create metal oxides. Waste metal from a steel mill would be recovered, and in order to be recycled, the zinc would need to be removed from any iron. Usually, the process involves removing the zinc by reacting it with oxygen in a furnace at 1500°C to create zinc oxide. But Jason’s team had discovered a way to create zinc oxide at near room temperature. They would use their ionic liquids to effectively corrode the zinc out of the iron into oxide nanoparticles. Nanomox was formed in 2020.
Jason points out that many of his companies share some serendipitous link with his life – one of his companies, DyeRecycle, extracts dyes out of textiles and recycles them, linking back to his own penchant for wearing brightly-dyed Hawaiian shirts.
Jason Hallett in Antarctica, holding a test tube of ionic liquid
Jason Hallett in Antarctica, holding a test tube of ionic liquid
He is now the co-founder of six companies with no intent on slowing down. He says researchers and students are hungrier than ever for entrepreneurial success.
It was 2008 when he was interviewing for an assistant professor lectureship position in the US. It had been two years since he left Georgia Tech and he was ready for a stable position as an academic. He still had a tendency to jump into new fields he had “no business in being” and turning problems on their head with his ‘engineer-ification’ approach: “I was accused of being a one-trick pony, I mean openly as well,” he says, “I would maybe say it was a fair statement – it’s a good trick, that’s all I can say in my defence.”
There were knock-on effects in acceptance that has helped turn our biorefinery research from impossible to almost pedestrian.
The biotechnology revolution changed how people would treat him: “There were knock-on effects in acceptance that has helped turn our biorefinery research from impossible to almost pedestrian. All of a sudden there’s mainstream acceptance where people say, ‘Of course that would work, it’s been known for 20 years,’ but 20 years ago I was called crazy.”
This cultural shift in finding market applications for academic research has meant chemical engineers like Jason have found themselves at the head of emerging industry. “I actually think that academics these days get a little too focused on developing a spin-out company,” he says, reflecting on recent trends in university spin-out spaces.
You can’t make the world a better place unless people actually use what you did, no matter how brilliant it is, if it’s a theory, that doesn’t have any direct impact.
But, for being a man with a hammer, Jason is intent on making the world a better place: “You can’t make the world a better place unless people actually use what you did, no matter how brilliant it is, if it’s a theory, that doesn’t have any direct impact.”
Creating a spin-out company, however, pushes a theory to its absolute limits. Rather than convincing industry to adopt a solution, why not bring that solution to market?
Lixea’s pilot plant located in Backhammar, Sweden, will process 100 tonnes of wood a year. It started as a small test tube processing just one gram of material.
Jason eventually brought that small test tube to Antarctica when he went there on holiday with his wife. The thought to bring a laboratory test tube with him was inspired by the unique photo opportunity: “I took an ionic liquid to Antarctica, so it could be the southern-most ionic liquid in human history,” he says, “But there I am in Antarctica wearing a Hawaiian shirt with the southernmost ionic liquid ever!”