The scientist and the donor
Why brain tissue donation remains vital to the advancement of Parkinson’s research
Parkinson's is a condition in which parts of the brain become progressively damaged over time. In the UK, around 145,000 people live with Parkinson’s and, globally, it is the fastest-growing neurological condition.
The scientific study of human brain tissue has led to breakthrough insights into how the condition progresses and has enabled the development of some essential treatments.
However, there is still no cure for Parkinson’s. While current treatments can help to target and manage symptoms in the short term, gaps in our understanding of the condition mean that there is ultimately no way of preventing or altering its progression.
The Parkinson's UK Brain Bank, located at Imperial College London’s Hammersmith Campus, is the world's only brain bank solely dedicated to Parkinson's research. The facility collects tissue from people with and without Parkinson's who have generously chosen to donate their brains to research.
Established in 2003, the Brain Bank is now home to almost 1,500 donated brains. Samples of the carefully preserved and catalogued tissue are shared with scientists around the world who are leading research into Parkinson’s and other related conditions.
The brain detective
Steve Gentleman is a Professor of Neuropathology and Director of the Parkinson's UK Brain Bank at Imperial.
Professor Gentleman's job, he explains, is "to be a kind of detective".
A typical Monday morning involves carefully inspecting and dissecting a donated brain: one half preserved and set aside for pathology studies, the other frozen for biochemistry and genetics research. He meticulously assesses the brain and documents the changes and pathology that have accumulated over the years.
"When you are holding the brain of a 70-year-old, you’re holding their whole life experience in your hands. Everything that has happened in their life is to some extent mirrored in what you’re looking at," he says.
"Essentially, I do an audit of all the changes in any one individual’s brain. Before we can send tissue out to researchers, they need to know everything that was going on in that person’s brain, because it may affect how they interpret their results.
"Everyone is individual and there are no two brains the same."

The Brain Bank
Over the past twenty years, the Parkinson's UK Brain Bank at Imperial has grown into a unique and valuable resource for researchers around the world.
"The Brain Bank is there to collect donations from people – both with and without Parkinson’s – who would like to donate their brain to research when the time comes. The donations we receive from people without the condition are essential to our work because they can be used as healthy controls in research experiments," says Professor Gentleman.
With support and funding from the charity Parkinson's UK, the Brain Bank team can collect brain tissue donations from anywhere across the UK and transport them safely to the facility in west London.
"When a donor has passed away, it’s often a family member or a health professional who will get in touch with us very quickly.
"We will then arrange the removal of the brain at a hospital somewhere in the country, whichever is the most local and available. The brain will either be couriered to us or collected by a technician and brought back to the facility at Hammersmith Hospital. It’s essential to do this within 48 hours to preserve the structural integrity of the brain.
"We usually receive a sample of the donor’s cerebrospinal fluid as well – this is enormously helpful in terms of biomarker research.
Members of the Brain Bank team, photographed at Imperial's Hammersmith Campus in 2018
Members of the Brain Bank team, photographed at Imperial's Hammersmith Campus in 2018
"Once the donations have arrived at the Brain Bank, we will preserve and characterise the tissue and work out what exactly went on in the brain of that individual. All of this information goes out with the tissue to research labs around the world.
"The two main questions we are looking to answer through research are: What is the disease process and how can we stop it? Can we find a useful biomarker to identify when an individual is developing Parkinson’s, before the appearance of symptoms?
"We will eventually have drugs that can stop the disease process - but we want to be able to treat people before they are symptomatic."
As well as helping scientists to better understand the pathology of the condition, the tissue and clinical information collected at the Brain Bank also support large-scale genetic analyses.
"This area of work is perhaps leading us towards personalised medicine, and working out why certain people may be more susceptible to developing Parkinson’s," says Professor Gentleman.
"In the future, this could mean that, by looking at your genetic makeup, you’ll be able to get a ‘risk assessment’ for developing Parkinson’s, as well as other conditions."
"When I asked Steve why someone would pledge, he simply said:
'We’re human and we want to help other people.'
That made me cry and realise that’s why I’m doing it too."

Rory's story
Rory Cellan-Jones is a technology journalist and author who, in 2019, was diagnosed with Parkinson’s. Following a visit to the Brain Bank at Imperial’s Hammersmith Campus in early 2020, Rory decided to pledge to donate his brain to research.
"For me, there are both personal and professional motivations for contributing to Parkinson’s research," he explains.
"Of course, as someone living with a Parkinson’s diagnosis, I’m eager to see progress towards new and better treatments, including some that could be available to me in my lifetime. And professionally, I have an interest in technology and how it can be used for good. I’ve taken part in a few trials that are looking at how apps and devices could help monitor and address Parkinson’s symptoms.
"In early 2020, the team at Parkinson’s UK asked if I’d like to visit the Brain Bank at Imperial and see if I’d consider pledging my grey matter to the cause.
"Donating my brain wasn’t something I’d considered before my diagnosis, and I certainly wouldn’t have known how to go about it had I not been invited to visit. Seeing how respectfully and skilfully the donations were treated, and hearing about how quickly each donation is ‘exhausted’ and how finite the resource is, made me realise just how important each and every donated brain is.
"I appreciate that not everyone will have the opportunity and privilege to see Professor Steve Gentleman at work, but that was what cemented my decision to donate my brain. It really brought home that we still don’t understand what is happening in the brain, whether it’s affected by Parkinson’s or not, and we need to address that in order to make meaningful progress toward better understanding and treatments.
"When I learned about the Brain Bank, I knew I wanted to donate and do my bit for medical research. But when the time came, I found it quite an emotional experience. When I asked Steve why someone would pledge, he simply said: 'We’re human and we want to help other people.' That made me cry and realise that’s why I’m doing it too.
Credit: Parkinson's UK
Credit: Parkinson's UK
"Each and every donation will be used to increase our understanding of Parkinson’s, why it happens and how it can be better treated or even stopped. That’s an incredible gift to be able to give to other people. Ultimately, we need these donations to bring us closer to a cure.
"Choosing to donate your brain to research is of course a big and personal decision that needs to be talked through with your family, but it could change the lives of millions of people. As I see it, when I’m eligible to donate, I won’t be using my brain anymore, so why not let researchers around the world put it to use one more time?"
A bigger pathological picture
There are still significant gaps in our understanding of how Parkinson's progresses through the brain. Figuring out the pathology of the condition is one of the key reasons why scientists need to study human brain tissue.
"Parkinson’s is different for everyone," Professor Gentleman explains.
"Most people associate it with movement problems. People are very familiar with a tremor - they’ve seen people with a shaking hand usually - but there’s also stiffness, rigidity or slow movement (bradykinesia). Any individual may have a collection of these symptoms.
"As time goes on, other things start happening as Parkinson's progresses in the brain. Problems with brain function begin and, unfortunately, they get worse as the disease progresses."
We do everything we can to treat the symptoms that we see, knowing that we are not actually stopping the underlying disease process.
Since the 1960s, scientists have known that a lack of the neurotransmitter dopamine in the brain plays a key role in these early symptoms. Sometimes referred to as a 'happiness hormone', dopamine also plays a vital role in regulating movement.
"From looking at human brain tissue, we’ve found that this loss of dopamine cells occurs in the area of the brain called the substantia nigra," says Professor Gentleman.
"Normally, this area contains large dopamine cells with a dark pigment in them called neuromelanin. The loss of these cells seems to be associated with the onset of movement problems that we see in Parkinson’s.
"For the past fifty or so years, the main treatment approach has been to try and replace the missing dopamine. We typically start by using something called L-DOPA, which helps the brain to produce more dopamine and maintain function. Most Parkinson’s patients tend to respond quite well to this initially.
"The reason why things don’t work out in the long term is that there’s much more going on in the brain than just the loss of these dopamine cells.
"The bottom line is that, at the moment, we don’t have any disease-course altering drugs. We do everything we can to treat the symptoms that we see, knowing that we are not actually stopping the underlying disease process.

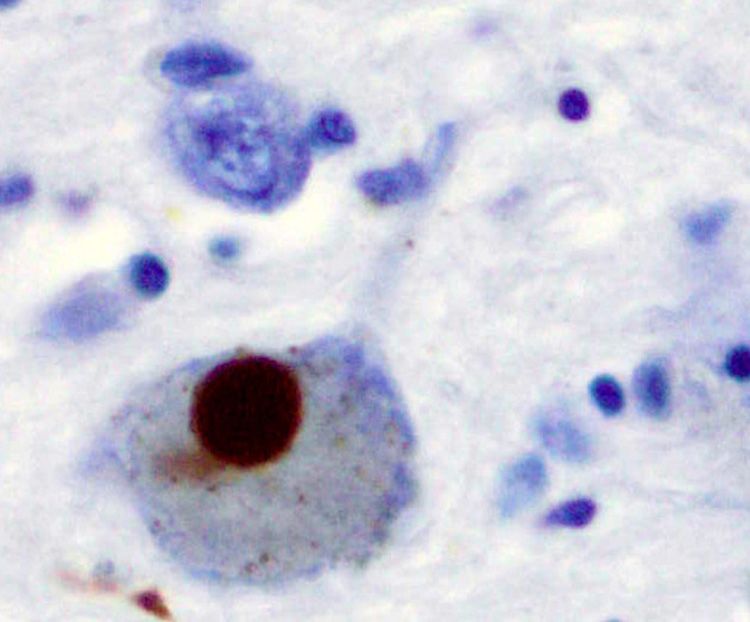
Alpha-synuclein stained brown in a Lewy body in the substantia nigra. Credit: Wikimedia images / Marvin 101.
Alpha-synuclein stained brown in a Lewy body in the substantia nigra. Credit: Wikimedia images / Marvin 101.
The problem protein
Parkinson's is relatively specific to humans meaning that, while disease processes can be modelled in animals, researchers need human brain tissue to fully validate their findings. Building on earlier breakthroughs, in the past 30 years this vital tissue has continued to provide scientists with important insights into the pathology of Parkinson's.
"Looking at human brain tissue is how we discovered that Parkinson’s is so much more than the death of dopamine cells," explains Professor Gentleman.
"The first big clue came in the late 1990s. Genetic analysis of families with Parkinson’s found that they carried a mutated gene for a protein called alpha-synuclein. Essentially, they had an inherited form of Parkinson’s."
Scientists then attempted to raise antibodies against this so-called problem protein. They examined brain tissue and found alpha-synuclein in Lewy bodies – complex, round inclusions in cells that are the characteristic pathological features of Parkinson's.
However, they also discovered that the protein had spread throughout the brain in end-stage Parkinson’s. Clearly, dopamine cells were just one part of the story.
"There was no way anybody could have worked this out without looking at actual human brain tissue," says Professor Gentleman.
Prior to this discovery, in 1985, scientists at the Karolinska Institute in Sweden made the first attempt to transplant dopamine-producing tissue in two patients with Parkinson's, and observed "some rewarding effects". Several larger clinical trials followed and, in many cases, demonstrated that these grafts could boost dopamine production and slow down the disease progression.
However, post-mortem examinations later revealed the spread of alpha-synuclein into the grafts, rendering them less effective.
"This discovery was important for two main reasons," explains Professor Gentleman.
"Firstly, it demonstrated that no amount of dopamine stops the underlying disease process. Secondly, it also provided us with new ideas about how alpha-synuclein pathology might be spreading throughout the brain.
Looking at human brain tissue is how we discovered that Parkinson’s is so much more than the death of dopamine cells
"At Imperial, one of the things we’re currently looking at is why the spread of alpha-synuclein through the brain is fairly predictable in Parkinson’s but varies in other neurological conditions. By looking at what happens in some of these rarer conditions, we can start to get a different picture of how things might be spreading through the brain in Parkinson’s.
"Looking at human brain tissue has also helped us to identify that there are different strains of disease caused by alpha-synuclein," he adds.
"Alpha-synuclein is not only a problem protein for Parkinson’s. It also plays a key role in a rare condition called multiple system atrophy (MSA), although it affects different cells.
"In animal models, it’s clear that you can transmit the type of alpha-synuclein seen in multiple system atrophy and subsequently see the disease pathology in the animals. However, the same approach doesn’t really work with the Parkinson’s-type alpha-synuclein.
"This tells us there’s something different at play – it’s a single protein, but there must be multiple forms. Depending on what form that protein is in, you’re going to see different clinical problems. This is really pushing us towards understanding more about the protein itself and these different strains.
"We can create a lab-made version of this protein to use in experiments. Interestingly, however, it doesn’t perform in the same way as alpha-synuclein extracted from a patient’s brain.
"Again, we wouldn’t have picked this up without using human tissue. We could have carried on performing experiments with our synthetic alpha-synuclein, but it’s much more subtle than that. "
Case study: Understanding toxic protein build-up in Parkinson's
Did you know that the brain has its very own 'clean-up' system to get rid of toxic proteins? The glymphatic system removes waste from the central nervous system, which in turn helps to keep the brain functioning properly.
We know that in Parkinson's, the protein alpha-synuclein builds up over time, causing the death of brain cells as it spreads.
Could a failure of the brain's waste clearance system be the cause of this toxic build-up?
UCL researchers Dr Ian Harrison and Sophie Llewellyn are studying human brain samples donated to Imperial's Brain Bank to answer this question.
Trip to @ImperialBrains this afternoon to pick up our tissue request from the @ParkinsonsUK Brain Bank. One very excited @SophieKLScience reading to get cracking with her experiments next week @CABI_UCL! 🧠🧪🧬 pic.twitter.com/LldZuAW6KX
— Ian Harrison (@IanH_Neurosci) March 25, 2022
As part of their Parkinson's UK-funded project, the team is aiming to find out if the water channels which help the glymphatic system clear waste proteins are affected by Parkinson’s.
They will look at donated brain tissue samples from individuals with early- and late-stage Parkinson’s to see whether there are any changes in the location and abundance of water channels lining blood vessels in the brain.
By looking at three different regions of the brain, and comparing them to samples from healthy controls, they will be able to see if and how these water channels are affected as the disease progresses.
"If this is the case, we’ll know to focus on this area of research for the development of a possible cure for Parkinson's," explains Dr Harrison, an Alzheimer’s Research UK / Parkinson’s UK Research Fellow at UCL's Centre for Advanced Biomedical Imaging.
"Our planned experiments represent the very first step towards this, as they will provide a greater understanding of the processes which occur as the disease progresses."
Choosing to donate your brain to research
So, what does the process look like for a prospective donor?
"When someone signs up to donate their brain tissue, they are added to our database and we obtain consent from both the individual and their nearest relatives," says Professor Gentleman.
"Pre-pandemic, we would frequently give tours of the facility to people who want to find out more about what we do here – we are starting to resume these, and people are very welcome to visit.
"Choosing to donate your brain to research is a big decision to make. There is support available and a great team who can talk through the whole process and answer any questions people might have. We know it’s a free choice and we try and facilitate it as best we can.
"The Brain Bank really is a world-leading resource, and we couldn’t do what we do without the generous contributions of donors."
- For more information about the Brain Bank and brain tissue donation, please visit the Brain Bank and Parkinson's UK websites