Contact
Professor Nagy Habib
nagy.habib@imperial.ac.uk
What we do
Translational research is at the heart of our research programme and we are constantly seeking new treatments for patients with liver and pancreatic cancer. We became interested in small activating oligonucleotides and its role with CEBPa gene. We are developing the mechanism of action with MiNA Therapeutics Limited, located at the Research & Innovation Hub at the White City Campus of Imperial College London (https://youtu.be/tk9NRtWaFT4).
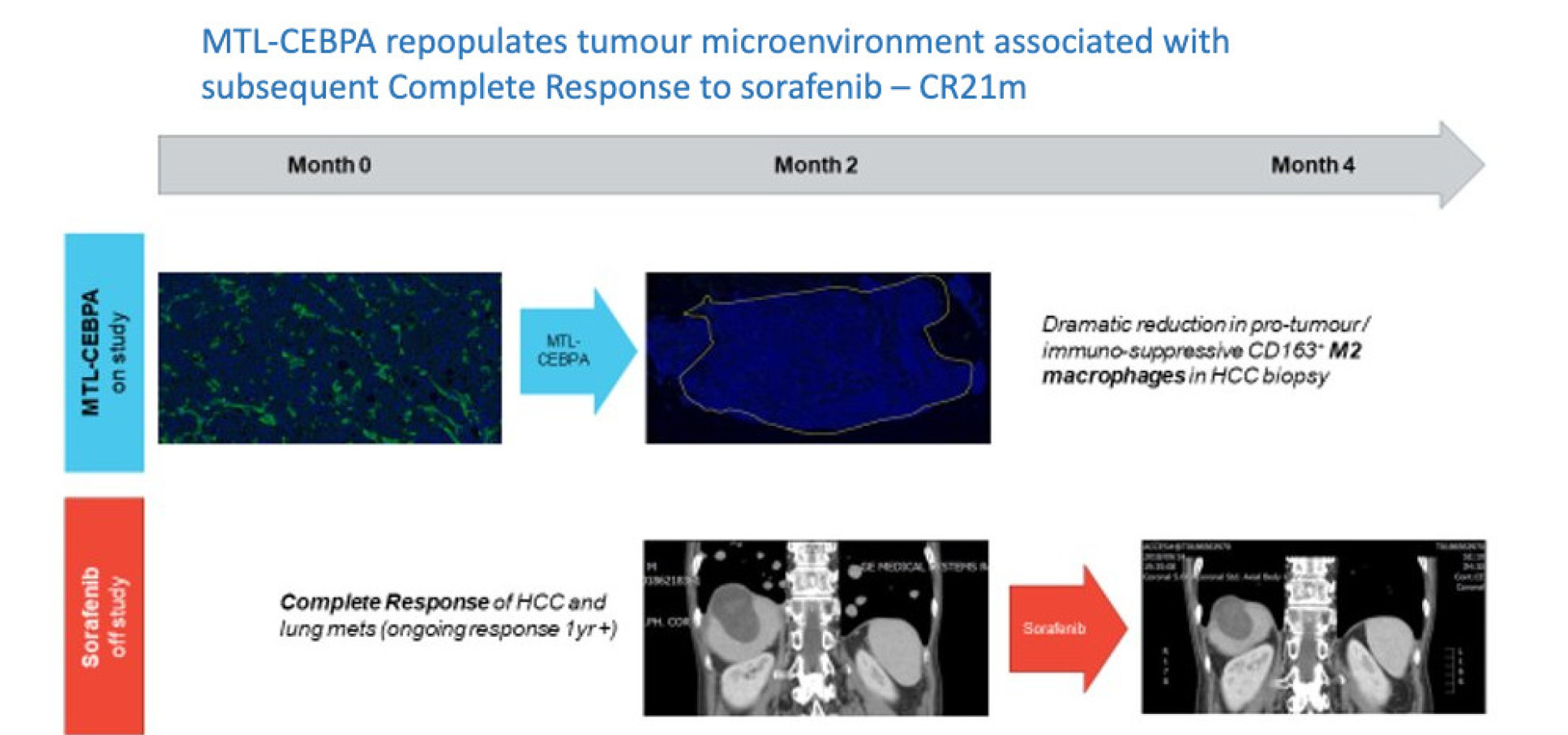
Preclinical data emerged suggesting that C/EBP-a effects on the tumour microenvironment through myeloid-derived suppressor cells could enhance response to sorafenib and check point inhibitors.
We planned, had approved, organised and carried out a Phase 1 dose escalation, dose expansion and dose combination study using the IMP MTL-CEBPA in patients with advanced liver cancer (OUTREACH Study; NCT:02716012; EudraCT: 2015-003051-21)
Why is it important
Only twenty per cent of the patients with HCC benefit from surgery. Therefore it is important to develop a new drug for the majority of inoperable patients, particularly given that liver cancer is increasing in frequency with the non-alcoholic steatohepatitis (NASH) epidemic.
Primary liver cancer is the seventh most common cancer in terms of incidence and fourth in terms of cancer-related mortality, globally accounting for more than 850,000 new cases annually and 9.1% of all cancer deaths. The majority (70%–90%) of patients with hepatocellular carcinoma (HCC) have a background of liver cirrhosis. Unfortunately, most patients are diagnosed with advanced disease as less than 20% of all patients with cirrhosis undergo screening. Sorafenib, a multikinase inhibitor, has been the first-line systemic treatment for HCC. However, the overall survival benefit with sorafenib in previously untreated patients with preserved liver function, good performance status, and advanced disease, although statistically significant, is disappointing (10.7 vs 7.9 months; ref. 3). In addition, lenvatinib was approved by the FDA as first-line treatment based on the REFLECT trial which showed noninferiority to sorafenib. Regorafenib, ramucirumab, and cabozantanib have demonstrated a further modest survival benefit in the second-line setting. The programmed cell death protein-1 (PD-1) immune checkpoint inhibitor.
How it can benefit patients
In 74 patients consecutively recruited in 8 centres in the UK, the drug was shown to be safe with no severe treatment-related adverse events. This drug [MTL-CEBPA] was the first in class and first in Human and showed efficacy in a subset group of patients that had no prior treatment with tyrosine kinase inhibitors and their cancer was of viral aetiologies. We were delighted to observe 20% complete response and 20% partial response, which is a higher response rated than the current approved standard of care.
Summary of current research
MTL-CEBPA is the first saRNA in clinical trials and demonstrates an acceptable safety profile and potential synergistic efficacy with TKIs in HCC. These encouraging phase I data validate targeting of C/EBP-a and have prompted MTL-CEBPA. Given these outcomes a second study using the same IMP, MTL-CEBPA, but combined with pembrolizumab has been started to test the drug combination in a variety of different solid tumour types (TIMEPOINT; NCT: 04105335: EudraCT: 2019-002231-28). An international Phase 2 of the OUTREACH study is in set up in 26 centres (OUTREACH2; NCT: 04710641; EudraCT: 2020-004378-23).
Our researchers
Professor Nagy Habib
/prod01/channel_3/media/migration/faculty-of-medicine/portraitjpgjsessionidjrg2gxrfmxxyp5gpjcjqym15ptm1cg6w31nbqytl13jfdc15tbvz371751212_1612180377479_x4.jpg)
Professor Nagy Habib
Professor of Hepatopancreatobiliary Surgery and Head of the Section
Mr Mikael Sodergren
/prod01/channel_3/media/migration/faculty-of-medicine/sapphire-bw-077-jpg-tojpeg-1563458519570-x1_1612180667117_x4.jpg)
Mr Mikael Sodergren
Senior Clinical Lecturer
Mr Duncan Spalding
/prod01/channel_3/media/migration/faculty-of-medicine/holding-png-tojpeg-1564655919889-x2_1612181984800_x4.jpg)
Mr Duncan Spalding
Clinical Research Fellow
Mr Madhava Pai
/prod01/channel_3/media/migration/faculty-of-medicine/thumbnail-img-5484_1613992440029_x4.jpg)
Mr Madhava Pai
Honorary Senior Lecturer
Dr Vikash Reebye
/prod01/channel_3/media/migration/faculty-of-medicine/portrait-5_1612182349887_x4.jpg)
Dr Vikash Reebye
Research Fellow in Gene Activation