Redox flow batteries (RFBs) are strong candidates for grid-scale energy storage due to their potential to decouple power and energy capacity. Commercial RFBs (e.g. all—vanadium system) have demonstrated attractive features, such as operation over a large number of cycles. However, RFBs have achieved limited market penetration due to issues related to cross-contamination, sluggish redox kinetics, low energy and power densities, and relatively high cost. The ESE group has been working on solving these problems with research focused on the development of novel hybrid (liquid/gas) RFB systems, study and optimisation of electrode microstructures and performance characterisation.
As part of the ESE group, the Brandon group at the Department of Earth Science and Engineering is leading this topic of research. The Brandon group primarily focuses on the development of cheaper and more efficient RFBs such as V/H2, PS/air and Mn/H2, and the study of the dominant transport and electrochemical processes affecting their performance. The group’s work combines a variety of experimental and simulation techniques such as polarisation and impedance spectra analysis, electrode design and manufacture using electrospinning, microstructure study via 3D tomography, flow field design and manufacture, battery optimisation using Gaussian processes, and performance modelling using reduced order and continuum modelling.
Current projects
Lead Researcher: Minnan Ye
PIs: Prof. Nigel Brandon, Prof Anthony Kucernak and Dr. Catalina Pino-Muñoz
Funding:
- Shell Global Solutions International B.V.
Regenerative fuel cells (RFCs) could offer pathways to reduce the costs of redox flow batteries. But their performances, such as energy efficiency and power density, might be required to be further improved to become competitive with the other electrical energy storage technologies. Modelling could help to simulate the RFB behaviours and reflect the issues addressed, which is an efficient and cheap tool to optimize the RFB performance and cost. The aim of the project is to develop a model, specific at the stack/system level, that could be used to investigate the performance or cost of different regenerative fuel cells, such as RHVFC, H2/BQDS RFC, H2/Mn RFC. The model is expected to be used for design optimization and control/monitoring-oriented applications of RFC system performance and cost.


Redox flow cell testing will be carried out in the Brandon laboratories at Imperial College.
Publications:
- In preparation
Past projects
- Polysulfide-air redox flow battery
- A unique approach to understanding relationship between electrode microstructure and RFB performance
- Reduced-order model for a regenerative hydrogen-vanadium fuel cell
Lead Researcher: Dr Yuhua Xia
PIs: Prof. Nigel Brandon
Funding:
- EPSRC UK grant, Lower Cost and Longer Life Flow Batteries for Grid Scale Energy Storage (EP/L014289/1)
- EPSRC UK grant, Energy Storage for Low Carbon Grids (EP/K002252/1 )
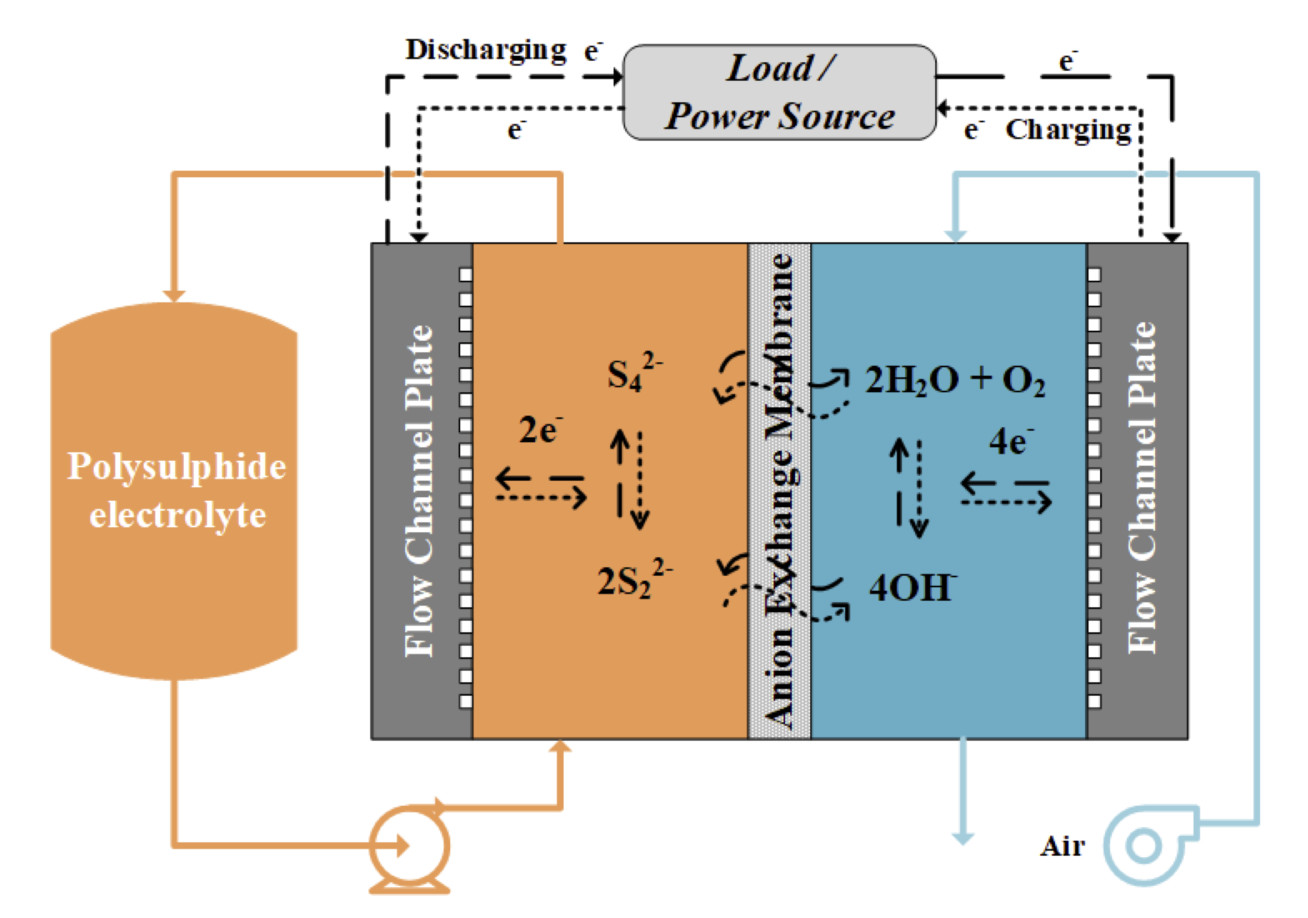
The aim of this project is to develop a low-cost redox flow battery for medium to large scale energy storage purposes. This polysulfide-air redox flow battery (PSA) utilises aqueous polysulfide redox couple (S42-/S22-) and O2/OH-redox couple on the negative and positive half-cells respectively, separated by an anion-exchange membrane (Figure 1). The main focus of this project is to develop highly functional and long-term stable aqueous polysulfide electrode, utilising transition metal sulfide catalysts. In addition, the development of suitable ion-exchange membrane and reversible air(oxygen) electrode are also conducted in collaboration with other research groups at Imperial College London. Experimental studies were carried out using a 5/25 cm2 redox flow battery test fixture, a 3-electrode cell, and a potentiostat.
Collaborators:
- ESE group - Earth Science and Engineering Department: Dr Mengzheng Ouyang and Mr Ben Simon
- Department of Chemistry: Prof. Anthony Kucernak
- Department of Materials: Dr Ifan Stephen
- Department of Chemical Engineering: Dr Qilei Song
- RFC Power Ltd.: Dr Ashkan Kavei
- Addionics Ltd.: Dr Vladimir Yufit
- University of Warwick: Dr Barun Chakrabarti
Publications:
-
Xia Y, Ouyang M, Yufit V, Tan R, Regoutz A, Wang A, Mao W, Chakrabarti B, Kavei A, Song Q, Kucernak AR, Brandon NP, 2022, A cost-effective alkaline polysulfide-air redox flow battery enabled by a dual-membrane cell architecture, Nature Communications, Vol: 13 (2388)
 Lead Researcher: Ben Simon
Lead Researcher: Ben Simon
PIs: Prof. Nigel Brandon and Prof Anthony Kucernak
Funding:
- EPSRC UK grant, Lower Cost and Longer Life Flow Batteries for Grid Scale Energy Storage (EP/L014289/1)
- Shell Global Solutions International B.V.
This international collaboration’s main aim is to understand and unpick the relationship between electrode microstructure and redox flow battery performance. The collaborators have taken a broad approach by using a wide range of analysis techniques such as X-ray computed tomography, electrochemical impedance spectroscopy and two types of model simulation, to separate out and decipher the dominant transport processes a redox flow battery system. The outcomes aim to be as far reaching and applicable as possible to liquid flow-through-type flow cells. Microstructural properties are shown to have unexpected and widely varying influences on the performance and certain properties are shown to have a significant impact on cell performance.
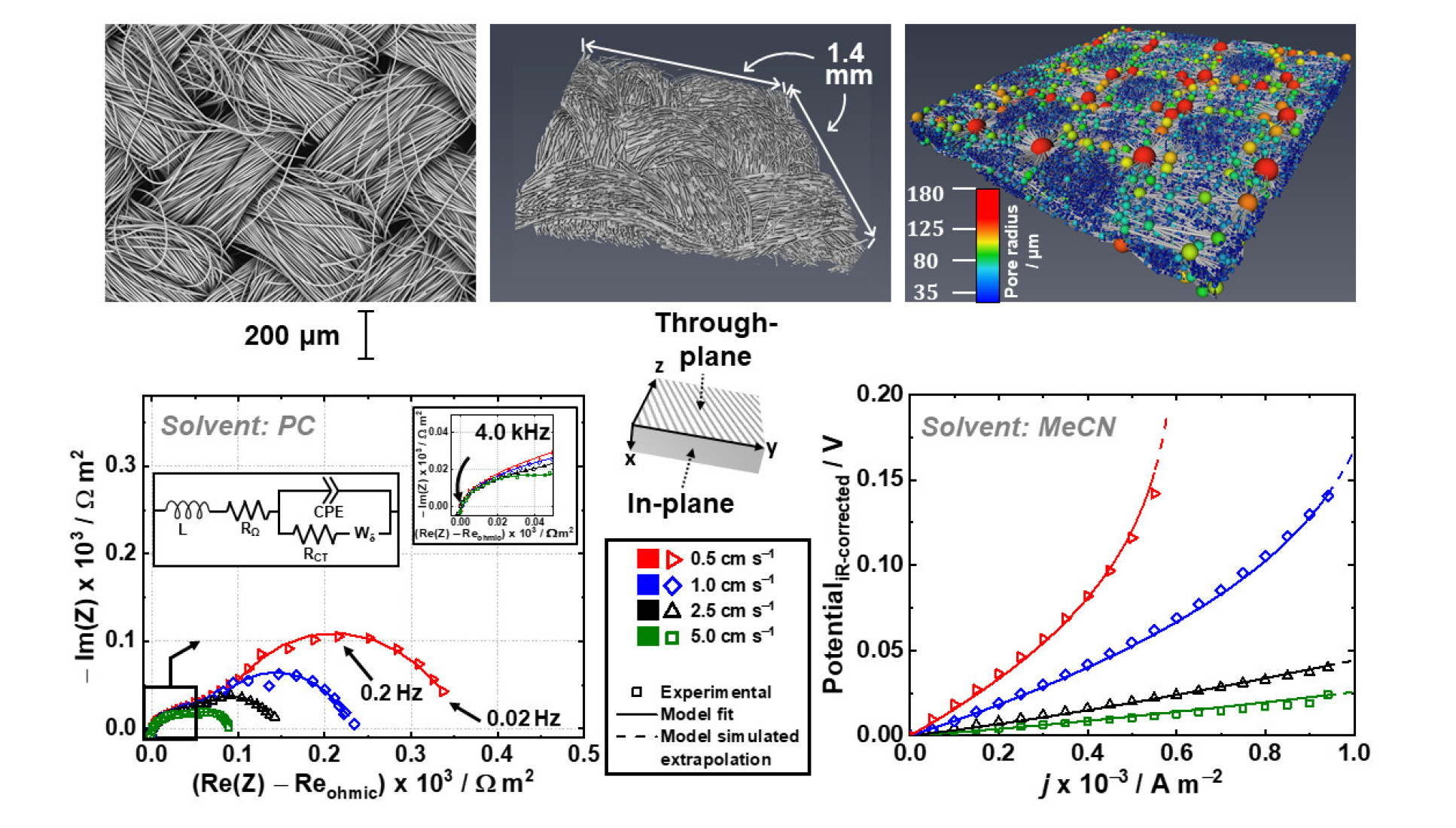
Redox flow cell testing was carried out in the Brushett laboratories at Massachusetts Institute of Technology where they have several devoted testing stations. A GE-Nanotom-S XCT machine was used for imaging analysis at Imperial College.
Collaborators:
- ESE group - Earth Science and Engineering Department: Andrea Gayon-Lombardo, Dr Catalina Pino-Muñoz and Dr Charles Wood
- ESE group - Dyson School of Design Engineering: Dr Sam Cooper
- Chemistry Department: Prof Anthony Kucernak
- Massachusetts Institute of Technology (MIT): Kevin Tenny, Katherine Greco and Prof Fikile Brushett
- Technical University of Eindhoven: Dr Antoni Forner-Cuenca
Publications:
- Simon BA, Gayon-Lombard A, Pino-Muñoz CA, Wood C, Tenny KM, Greco KV, Cooper SJ, Forner-Cuenca A, Brushett FR, Kucernak AR, Brandon NP, 2021, Combining electrochemical and imaging analyses to understand the effect of electrode microstructure and electrolyte properties on redox flow batteries, Applied Energy, Vol: 306, Part: 2, 117678
- Thesis: Electrode, Electrocatalyst and Electrolyte Development for Hybrid Redox Flow Batteries, Dr Benedict Simon, 2022
Lead Researcher: Dr Catalina Pino-Muñoz
PI: Prof. Nigel Brandon
Funding:
- EPSRC UK grant, SUPERGEN Energy Storage Hub (EP/L019469/1)
- EPSRC UK grant, Energy Storage for Low Carbon Grids (EP/K002252/1)
- EPSRC UK grant, Lower Cost and Longer Life Flow Batteries for Grid Scale Energy Storage (EP/L014289/1)
- 'Becas Chile' scholarship from the Chilean National Commission of Science and Technology (CONICYT)
A regenerative hydrogen-vanadium fuel cell (RHVFC) is of interest for grid-scale energy storage due to its fast-reversible kinetics and the facile separation of crossover species if present. The overall system cost is reduced by utilising only half of the vanadium required for an all-vanadium battery. However, there are challenges related to scale-up and optimisation. Better performance could be obtained by studying component materials, design and operating conditions, to which modelling and simulation are an indispensable tool. This project aim is to investigate the interplay of various physicochemical phenomena involved in an RHVFC, distinguishing the relevant physical and electrochemical processes.
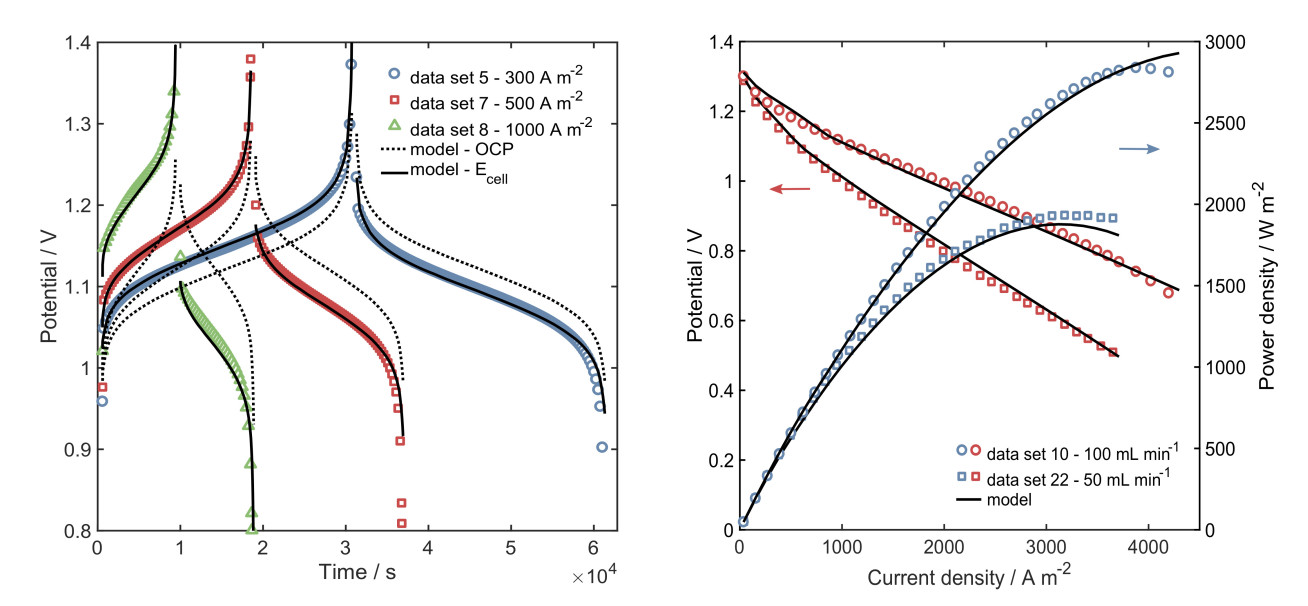
The main outcomes of the projects include a reduced-order model based on the physicochemical phenomena explained by mass conservation, transport mechanisms and electrochemical processes; and, a thorough experimental characterisation of the cell performance including a study of the effect of properties and operating conditions (Figure 1). Simulation results showed that the crossover of ionic species (capacity loss) is a limiting factor during long cycling operation. A simplified crossover model was included in the model and encouraging results were obtained. This topic is the interest of a project currently undergoing.
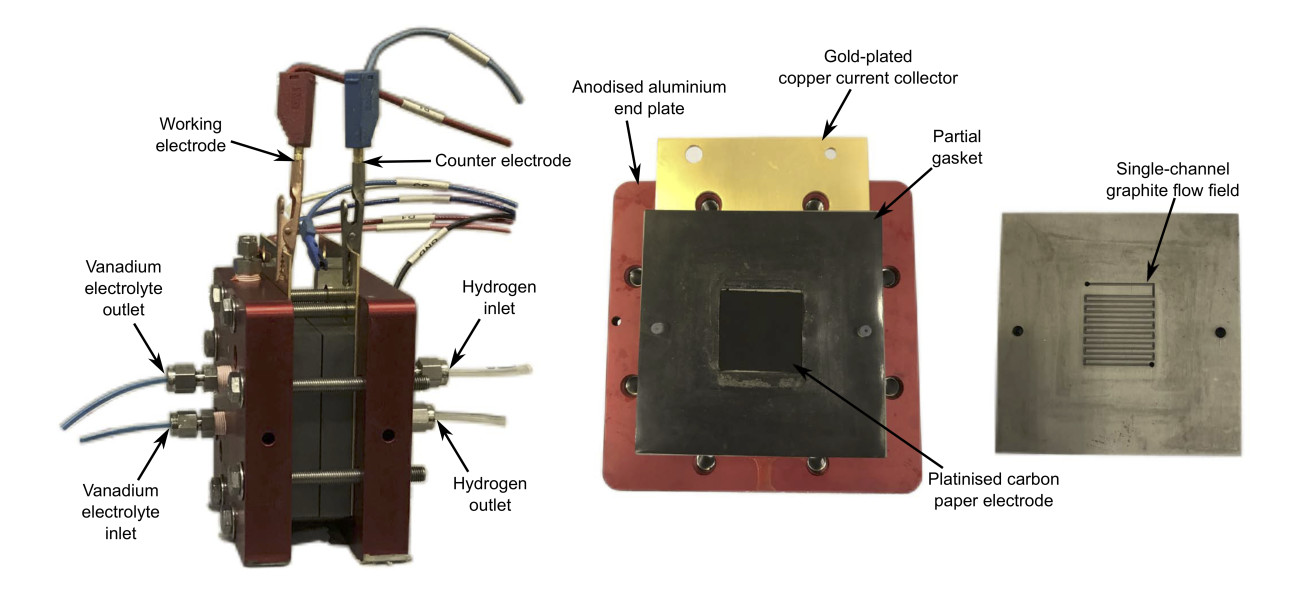
Hydrogen-vanadium cell testing was carried out in the Brandon laboratories at Imperial College London. Two cell systems were employed: (1) a 25 cm2 in-house manufactured cell, and (2) a 5 cm2 commercial (Scribner Associates) cell (Figure 2). A Bio-Logic potentiostat (VSP-300) running EC-Lab software was used for the electrochemical testing, including electrochemical impedance spectroscopy (EIS), open circuit potential (OCP), galvanostatic charge and discharge, power curves and cycling tests. Additionally, a GE-Nanotom-S XCT machine was used to obtain images of the cathodic carbon electrode (Figure 3).
Collaborators:
- Addionics: Dr Vladimir Yufit
- University of Warwick: Dr Barun Chakrabarti
- M-KOPA Solar: Dr Harini Hewa Dewage
Publications:
- Pino-Muñoz CA, Chakrabarti BK, Yufit V, Brandon NP, 2019, Characterization of a regenerative hydrogen-vanadium fuel cell using an experimentally validated unit cell model, Journal of the Electrochemical Society, Vol: 166, Pages: A3511-A3524, ISSN: 0013-4651
- Pino-Muñoz CA, Dewage HH, Yufit V, , 2017, Unit cell model of a regenerative hydrogen-vanadium fuel cell, Journal of the Electrochemical Society, Vol: 164, Pages: F1717-F1732, ISSN: 0013-4651
- Thesis: Mathematical modelling of vanadium-based redox flow batteries, Dr Catalina Pino-Munoz, 2019.
Recent publications 2020 - to date
Xia Y, Ouyang M, Yufit V, Tan R, Regoutz A, Wang A, Mao W, Chakrabarti B, Kavei A, Song Q, Kucernak AR, Brandon NP, 2022, A cost-effective alkaline polysulfide-air redox flow battery enabled by a dual-membrane cell architecture, Nature Communications, Vol: 13 (2388)
Simon BA, Gayon-Lombardo A, Pino-Muñoz CA, Wood CE, Tenny KM, Grecoc KV, Cooper SJ, Forner-Cuenca A, Brushett FR, Kucernak AR, Brandon NP, 2021, Combining electrochemical and imaging analyses to understand the effect of electrode microstructure and electrolyte properties on redox flow batteries, Applied Energy, Vol: 306, Part: 2, 117678
Zhang D, Forner-Cuenca A, Taiwo OO, Yufit V, Brushett FR, Brandon NP, Gu S, Cai Q, 2020, Understanding the role of the porous electrode microstructure in redox flow battery performance using an experimentally validated 3D pore-scale lattice Boltzmann model, Journal of Power Sources, Vol: 447.

