Colour-change urine test for cancer shows potential in mouse study

Mouse urine became blue in the presence of colon tumours
A simple and sensitive urine test developed by Imperial and MIT engineers has produced a colour change in urine to signal growing tumours in mice.
Tools that detect cancer in its early stages can increase patient survival and quality of life. However, cancer screening approaches often call for expensive equipment and trips to the clinic, which may not be feasible in rural or developing areas with little medical infrastructure.
By taking advantage of this chemical reaction that produces a colour change, this test can be administered without the need for expensive and hard-to-use lab instruments. Professor Molly Stevens Departments of Materials and Bioengineering
The emerging field of point-of-care diagnostics is therefore working on cheaper, faster, and easier-to-use tests. An international pair of engineering labs are championing this approach and have developed a tool that changes the colour of mouse urine when colon cancer, also known as bowel cancer, is present.
The findings are published in Nature Nanotechnology.
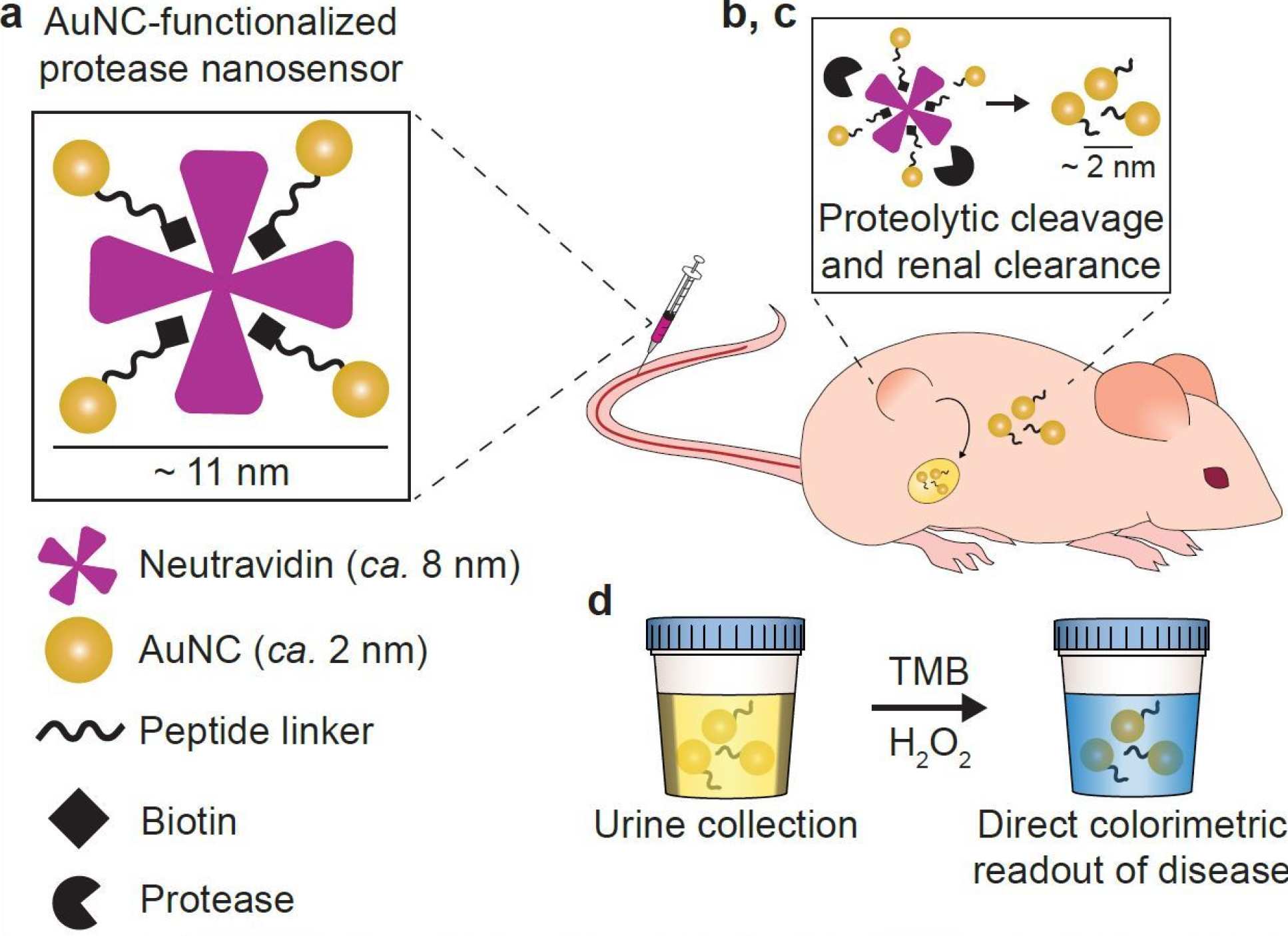
The early stage technology, developed by teams led by Imperial’s Professor Molly Stevens and MIT's Professor Sangeeta Bhatia, works by injecting nanosensors into mice, which are cut up by enzymes released by tumours known as proteases.
When the nanosensors are broken up by proteases, they pass through the kidney, and can be seen with the naked eye after a urine test that produces a blue colour change.
The researchers applied this technology to mice with colon cancer, and

found that urine from tumour-bearing mice becomes bright blue, relative to test samples taken from healthy mice.
Professor Stevens, of Imperial’s Departments of Materials and Bioengineering, said: “By taking advantage of this chemical reaction that produces a colour change, this test can be administered without the need for expensive and hard-to-use lab instruments.
“The simple readout could potentially be captured by a smartphone picture and transmitted to remote caregivers to connect patients to treatment.”
Sensing signals
When tumours grow and spread, they often produce biological signals known as biomarkers that clinicians use to both detect and track disease.
By designing versions of our sensors that can be cut by different proteases, we could apply this colour-based test to detect a diversity of conditions. Ava Soleimany MIT
One family of tumour enzymes known as matrix metalloproteinases (MMPs) help promote the growth and spread of tumours by 'chewing up' the tissue scaffolds that normally keep cells in place.
Many cancer types, including colon tumours, produce high levels of several MMP enzymes, including one called MMP9.
In this study, the Imperial-MIT team developed nanosensors where ultra-small gold nanoclusters (AuNCs) were connected to a protein carrier called neutravidin, through linkers that are broken by MMP9s
To develop the colour-changing urine test, the researchers used two AuNC properties – their very small (<2 nanometre) size, and their ability to cause a blue colour change when treated with a chemical substrate and hydrogen peroxide.
The researchers designed the AuNC-protein complexes to disassemble after being cut by MMPs in the tumour environment or blood. When broken apart, the released AuNCs travel via the blood to the kidneys, where tehy are small enough to be filtered through and into the urine.
In healthy mice without high MMP levels, the complexes remain intact, and are too large to pass into the urine. If AuNCs have been concentrated in the urine, a chemical test will produce a blue colour change that is visible with the naked eye.
For this study, the researchers developed sensors that are cut apart by particular MMPs and tested them in mice. The researchers demonstrated that their colour change test could accurately detect which urine samples came from mice with colon tumours in a study of 28 mice injected with the sensors, where 14 mice were healthy and 14 had colon tumours.
Within half an hour of the chemical treatment, only the urine from mice with colon tumours had a strong blue colour. By contrast, urine from the healthy control mice exhibited no colour change.
The team also designed the AuNC surfaces to go ‘unseen’ by the immune system to prevent immune reactions or toxic side effects, and to prevent abundant serum proteins from sticking to them, which would make the nanosensors too large to be filtered by the kidneys.
During a four-week follow up after nanosensor administration, the mice showed no signs of side effects, and there was no evidence that the protein-sensor complex or free AuNCs lingered in the bodies of the mice.
Co-first author Dr Colleen Loynachan, of Imperial’s Department of Materials, said: “The AuNCs are similar to materials already used in the clinic for imaging tumours, but here we are taking advantage of their unique properties to give us additional information about disease. However, there’s still a lot of optimisation and testing needed before the technology can move beyond the lab.”
Accessible diagnostics
Next, the team will work to increase the specificity and sensitivity of the sensors by testing them in more animal models to investigate diagnostic accuracy and safety.
Co-first author Ava Soleimany, of MIT, said: “Proteases play functional roles in a number of diseases such as cancer and infectious diseases. By designing versions of our sensors that can be cut by different proteases, we could apply this colour-based test to detect a diversity of conditions.”
The researchers are now working on a formulation that is easier to administer, and identifying ways to make the sensors responsive to multiple biomarkers in order to distinguish between cancers and other diseases.
This work was funded by the Engineering and Physical Sciences Research Council (UK), the Wellcome Trust (UK), European Research Council, the National Cancer Institute (USA), the National Institutes of Health (USA), the National Institute of Environmental Health Sciences (USA), the National Science Foundation (USA), the Ludwig Fund for Cancer Research, the Koch Institute Marble Center for Cancer Nanomedicine, the Howard Hughes Medical Institute, and the Swiss National Science Foundation.
“Renal clearable catalytic gold nanoclusters for in vivo disease monitoring” by Colleen N. Loynachan, Ava P. Soleimany, Jaideep S. Dudani, Yiyang Lin, Adrian Najer, Ahmet Bekdemir, Qu Chen, Sangeeta N. Bhatia and Molly M. Stevens, published 2 September 2019 in Nature Nanotechnology.
All image credits to Imperial College London/MIT
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Caroline Brogan
Communications Division