Structure, mechanism, and inhibition of Hedgehog Acyltransferase
Collaborative research helps to elucidate the structure and function of membrane-bound protein implicated in cancer
Researchers in the group of Prof. Ed Tate in the Department of Chemistry have recently published a new paper entitled “Structure, mechanism, and inhibition of Hedgehog Acyltransferase” in Molecular Cell, in close collaboration with the lab of Prof. Christian Siebold at the University of Oxford.
The hedgehog (Hh) pathway is a crucial regulator of embryonic development, but it is also a driver in a variety of cancers with up to 25% of all cancer-related deaths involving disruptions in Hh signalling. The Hh pathway is thus an important and sought-after target in cancer.
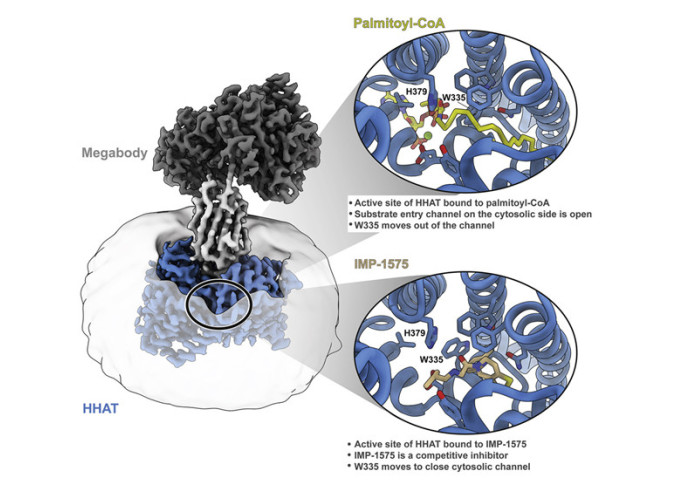
Hh proteins, which carry the Hh pathway signal between a ‘producing’ cell and a ‘receiving’ cell, are specifically modified in the cell with two lipids which are critical for signalling: a C-terminal cholesterol and an N-terminal palmitate. Palmitoylation is catalysed by Hedgehog Acyltransferase (HHAT), a Membrane Bound O-Acyltransferase (MBOAT) enzyme that resides in the ER-membrane. HHAT inhibitors can block Hh signalling and represent novel potential anticancer drugs. However, obtaining structural information for membrane proteins is notoriously difficult and data on the MBOAT family of proteins is scarce, limiting the ability to design HHAT-targeting drugs.
The authors describe the cryogenic electron microscopy (cryo-EM) revealing the first structure of HHAT bound to an inhibitor, IMP-1575, previously reported by the Tate lab as the most potent HHAT inhibitor reported to date, alongside a structure bound to a non-hydrolysable analogue of its co-substrate palmitoyl coenzyme A. The structures revealed several surprises, including a bound iron-haem complex which stabilises the protein fold, and a novel pocket which appears to bind a cholesterol molecule. This led the authors to speculate that HHAT might bind the cholesterol modification of Hh, a feature unique to the Hh pathway.
Prof. Ed Tate, who directed the study together with Prof. Christian Siebold, said: “This unique structure provides us with the first opportunity to rationally design inhibitors against HHAT, opening the door to the generation new drugs inhibitors, and a new dimension to the field of Hh inhibition in cancer, potentially impacting real lives.”
Dr Sebastian Andrei, a Marie Curie Fellow in the Tate group and joint first author on the paper, continues: “Our results also give insight into burning questions regarding the mechanism of this intriguing enzyme, including what the exact mechanism of the palmitoylation reaction is, and how palmitoylated Hh proteins might exit the active site following the reaction.”
The work was supported by a wide range of funders, including a Cancer Research UK Programme Foundation Award to Prof. Tate, a BBSRC award to Profs Tate and Siebold, and a European Union Horizon 2020 Marie Curie Fellowship to Dr Andrei. The research was co-led by Dr Sebastian Andrei and Tate group alumnus Dr Tom Lanyon-Hogg, who now runs an independent research group at the University of Oxford.
Congratulations to everyone involved!
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Ravi Singh
Department of Chemistry