Potent HHAT inhibitor offers chance to understand hedgehog signaling in disease
The Tate lab has disclosed a hit optimization campaign that found the most potent HHAT inhibitor reported so far, a new tool for investigating disease
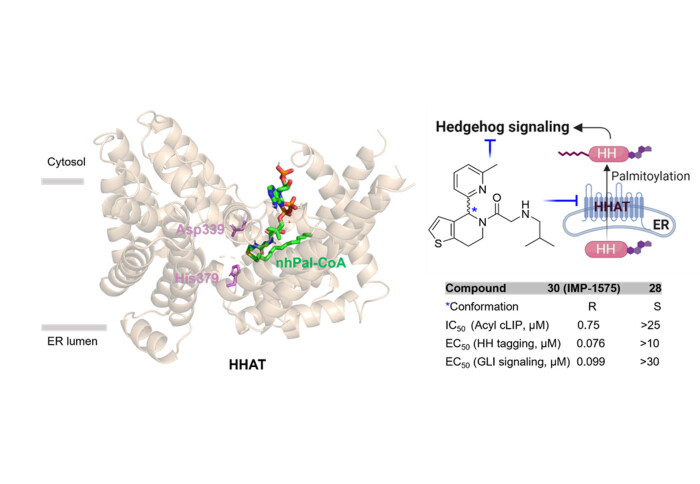
Hedgehog signaling is important for embryonic development, but it is also reactivated during initiation and progression of many cancers. Functional activity of secreted Hedgehog signaling proteins requires their N-palmitoylation at the N-terminus by the palmitoyl transferase Hedgehog acyltransferase (HHAT). The Tate lab has a long-standing interest in targeting HHAT as a potential cancer drug target, since it is required to enable Hedgehog signaling. Our previous work provided structural information on the mechanism of HHAT-catalyzed palmitoylation and the binding mode of HHAT inhibitor IMP-1575 (Molecular Cell 2021, 81, 5025).
The latest publication in the Journal of Medicinal Chemistry reports a systematic structure-activity relationship (SAR) study leading to the potent HHAT inhibitor IMP-1575. Based on structural data, we designed 50 analogues and characterized them in enzymatic and cellular assays, identifying several potent analogues with IC50 down to the nanomolar range against purified HHAT, which also inhibit Sonic Hedgehog (SHH) palmitoylation in cells and suppress the SHH signaling pathway. Cellular assays show that IMP-1575 has no detectable off-target cell toxicity across the range at which it inhibits HHAT in cells, and inhibits the palmitoylation of SHH as well HH signaling with nM potency in cells. The opposite ((S)-configured) enantiomer shows no HHAT inhibition in both enzyme and cellular assays and, in combination with IMP-1575, provides a powerful pair of tool molecules for investigation of HHAT activity in cells. However, the in vivo stability of this chemical series is not suitable for progression to preclinical development. Therefore, we have undertaken a high-throughput screen to identify multiple novel HHAT inhibitor series with much higher potency and metabolic stability. We expect that the first results from this work will be reported later in 2024.
This work was the result of a multidisciplinary collaboration between Imperial College London, the University of Oxford and the Institute of Cancer Research, and was supported by Cancer Research UK, the European Research Council, the BBSRC, the UKRI, the European Union Horizon 2020 programme and the Wellcome Trust and European Commission’s Research Executive Agency.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Edward Bartlett
Department of Chemistry