STED microscopy involves exciting a fluorescently labelled sample in a laser scanning confocal microscope and then introducing a second depletion laser beam that is collinear to the excitation beam. The wavelength of this depletion beam should be tuned within the emission spectrum of the fluorophore and above the excitation wavelength such that it stimulates emission and depletes the excited state population of the fluorophore, thereby effectively “switching off” the florescence emission. To increase the lateral resolution beyond the diffraction limit, the depletion beam is contrived to have an annular spatial profile that is zero on the optical axis but otherwise presents high intensity where it overlaps the excitation beam. The detected fluorescence emission will therefore be at a maximum on axis at the centre of the beams but suppressed elsewhere according to the intensity of the depleting radiation. As the depletion beam intensity is increased, the region of undepleted fluorophores decreases around the centre where the depletion intensity is zero. This reduced excitation region can be scanned across the sample to produce super-resolved images with a lateral resolution given by
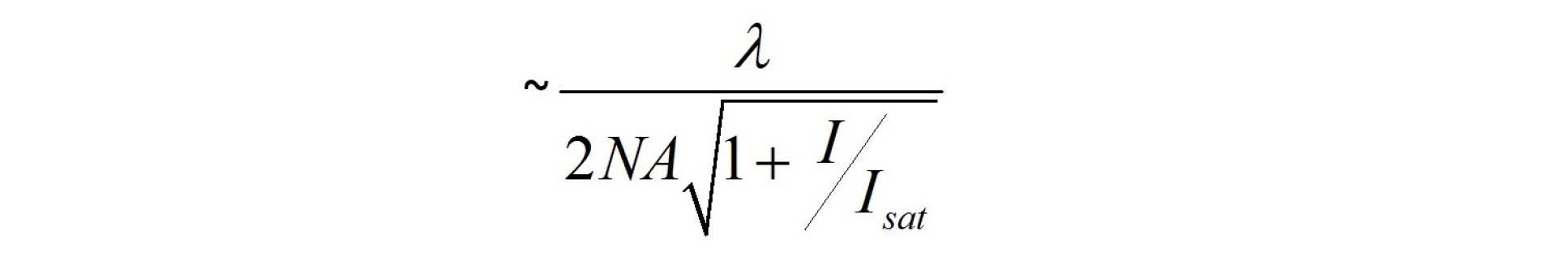
where I is the intensity of the depletion beam and Isat is the saturation intensity of the fluorophore. A similar approach can be implemented to improve the axial resolution beyond the diffraction limit by contriving the depletion beam to have an intensity distribution that is high above and below but zero in the focal plane1.
STED microscopy was initially implemented with organic dye fluorophores and has subsequently been implemented with genetically expressed fluorescence proteins2. It has been widely applied to achieve sub-100 nm resolution in fixed and live biological samples, including of synaptic vesicles in living neurons at video rate3. Because it is based on confocal microscopy, it is more robust against background light than stochastically-switched single molecule localisation techniques and is able to image deeper inside biological tissue. To date it has already been realised in live microorganisms4 and mice5.
One drawback of STED microscopy is that the depletion beam is required to be sufficiently intense to deplete the excited fluorophore population and the achievable resolution depends in the incident power. These required power levels can lead to photobleaching and phototoxicity, as well as unwanted nonlinear optical effects. RESOLFT techniques implemented with photoswitchable fluorophores rather than depletion of fluorophore excitation can be realised at much lower powers. We are working to implement STED with lower depletion powers using plasmonic fluorophores comprising metallic nanoparticles loaded with dye – for which the plasmonic resonance increases the depletion for a given STED beam intensity6.
1 Klar, T. A., Jakobs, S., Dyba, M., Egner, A. and Hell, S. W., Proceedings of the National Academy of Sciences of the United States of America, 97 (2000) 8206
2 Hein, B., Willig, K. I. and Hell, S. W., Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 14271
3 Westphal, V., Rizzoli, S. O., Lauterbach, M. A., Kamin, D., Jahn, R. and Hell, S. W., Science, 320 (2008) 246
4 Rankin, B. R., Moneron, G., Wurm, C. A., Nelson, J. C., Walter, A., Schwarzer, D., Colo, D. A., Schroeder, J., Colón-Ramos, D. a. and Hell, S. W., Biophysical Journal, 100 (2011) L63
5 Berning, S., Willig, K. I., Steffens, H., Dibaj, P. and Hell, S. W., Science (New York, N.Y.), 335 (2012) 551
6 Experimental Proof of Concept of Nanoparticle-Assisted STED, Y. Sonnefraud, H. G. Sinclair, Y. Sivan , M. R. Foreman , C. W. Dunsby, M. A. A. Neil, P. M. French, and S. A. Maier, Nano Lett., 14 (2014), (8) 4449–4453, DOI: 10.1021/nl5014103