We have worked with Kentech Instruments Ltd since 1996 to develop wide-field time-gated imaging technology and this has resulted in what we believe to be a robust platform for FLIM-based studies that provides lifetime resolution down to below 100ps at time-gated image acquistion rates as fast as 10 frames/second. The latest generation of gated optical image intensifier (GOI) technology enables rapid wide-field time-gated FLIM with spatial resolution up to ~20 line pairs/mm.
We have applied this wide-field time-gated FLIM technology to super-resolved FLIM microscopy, to FLIM high content analysis and to optical FLIM tomography - both optical projection tomography (FLIM OPT) and diffuse fluorescence tomography.
Wide-field time-gated fluorescence lifetime imaging microscopy
The figure below shows a generic configuration for wide-field time-gated FLIM comprising the core elements : (i) ultrafast excitation source, (ii) GOI and computer-controlled delay generator, (iii) computer for data acquisition and analysis, and (iv) the optical instrument. Here the optical instrument is a wide-field epifluorescence microscope and the excitation radiation is homogenized using a holographic flat-top diffuser that is rotated to time-average speckle.
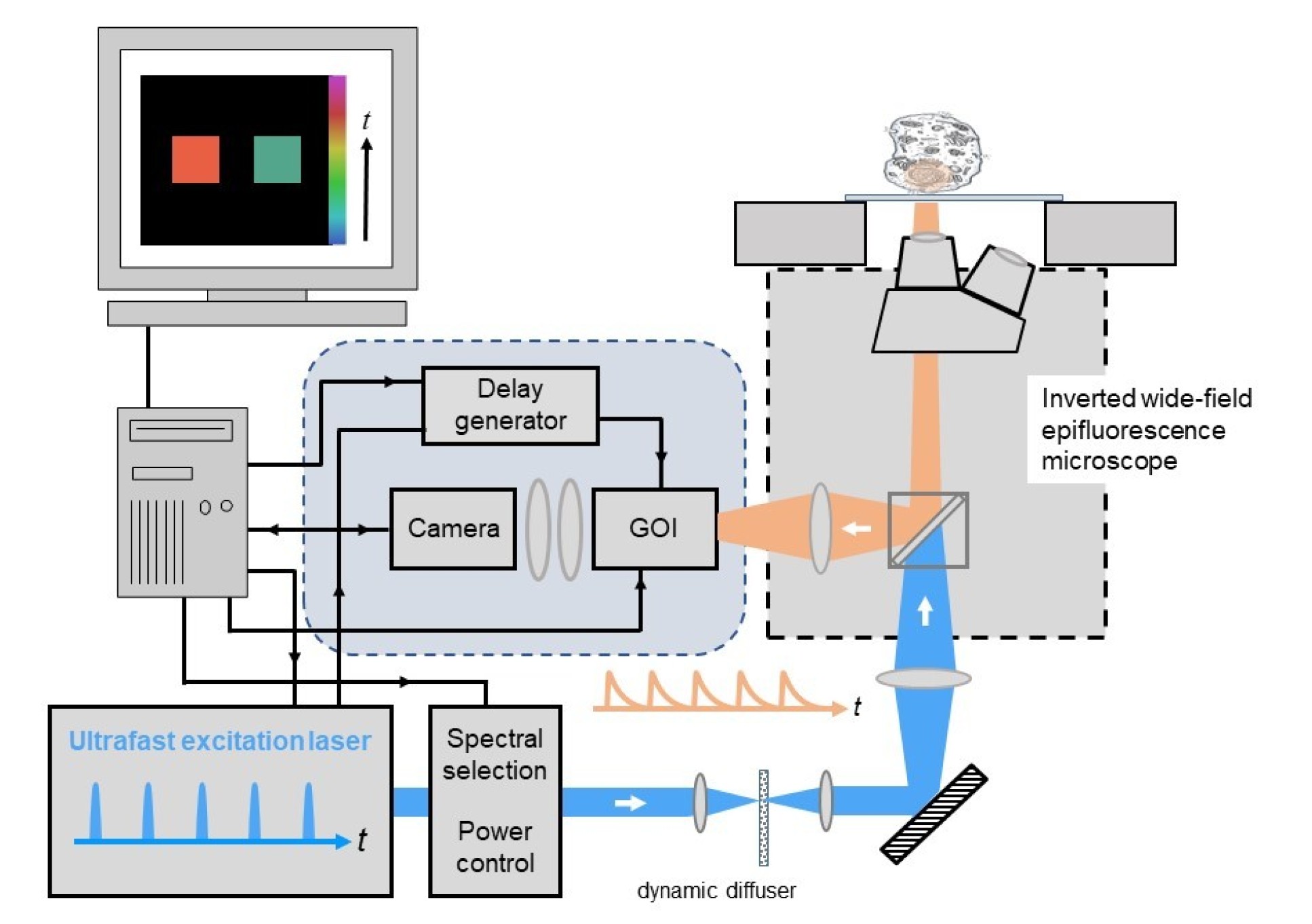
For the ultrafast laser source, we currently use a fibre-laser pumped supercontunuum source (e.g. NKT SuperK Fianium FIU-15 PP) or a femtosecond mode-locked Ti:Sapphire laser (e.g. Spectra-Physics, model Mai-Tai). We have also used gain-switched picosecond diode lasers (e.g. PicoQuant GmbH, model LDHP-C-440M with driver PDL-800-B) or a frequency-tripled Nd:YVO4 laser (Spectra-Physics, model Vanguard 350-HMD355) to provide excitation at 355 nm. The pulse repetition rate of the ultrafast excitation source should ideally be more than five times longer than the lifetimes to be measured, in order to avoid temporal overlap of fluorescence decay profiles excited by consecutive pulses. An ultrafast supercontinuum source with adjustable pulse repetition rate is therefore a particulary versatile excitation source for FLIM of a wide range of samples. When using ultrafast lasers with 80MHz pulse repetition rate for FLIM of samples labelled with typical fluorescent proteins or dyes, the FLIM analysis software must be able to take account of the overlapping fluorescence decay profiles.
To realise a uniform illumination intensity across the field of view for wide-field FLIM microscopy, the Gaussian intensity profiles of an excitation laser beam can be flattened using a "top-hat" holographic diffuser, which can be rotated or dithered (at 10-50 Hz) to time-average laser speckle in the illumination. This diffuser is imaged to the back focal plane of the microscope objective lens.
To realise time-gated imaging, our FLIM instrumentation is designed to use a GOI (Kentech Instruments Ltd, model HRI) and a computer-controlled delay generator (Kentech Instruments Ltd, agile delay unit). A cooled camera is required to read out the time-gated images from the phosphor of the GOI, for which we currently use a scientific CCD camera (e.g. Teledyne QImaging, model Retiga R1).
Wide-field optically sectioned time-gated fluorescence lifetime imaging microscopy
If optically sectioned FLIM is required, wide-field time-gated FLIM can be integrated with optical sectioning using a Nipkow spinning disc scanner, for which we currently use a Yokogawa CSU-X or CSU-W1). This can provide quasi-confocal FLIM at frame rates up to 10Hz and is particularly useful for quantitative live cell FLIM since the high degree of parallelisation (e.g. compared to laser scanning microsopy) greatly reduces the instantaneous light dose at the sample and so reduces photobleaching and phototoxocity. The instrument configuration is similar to that described above but the excitation light is coupled to the spinning disc scanner via a polarisation-preserving single mode optical fibre, as depicted below, and the GOI is mounted at the camera port of the Nipkow disc scanning unit.
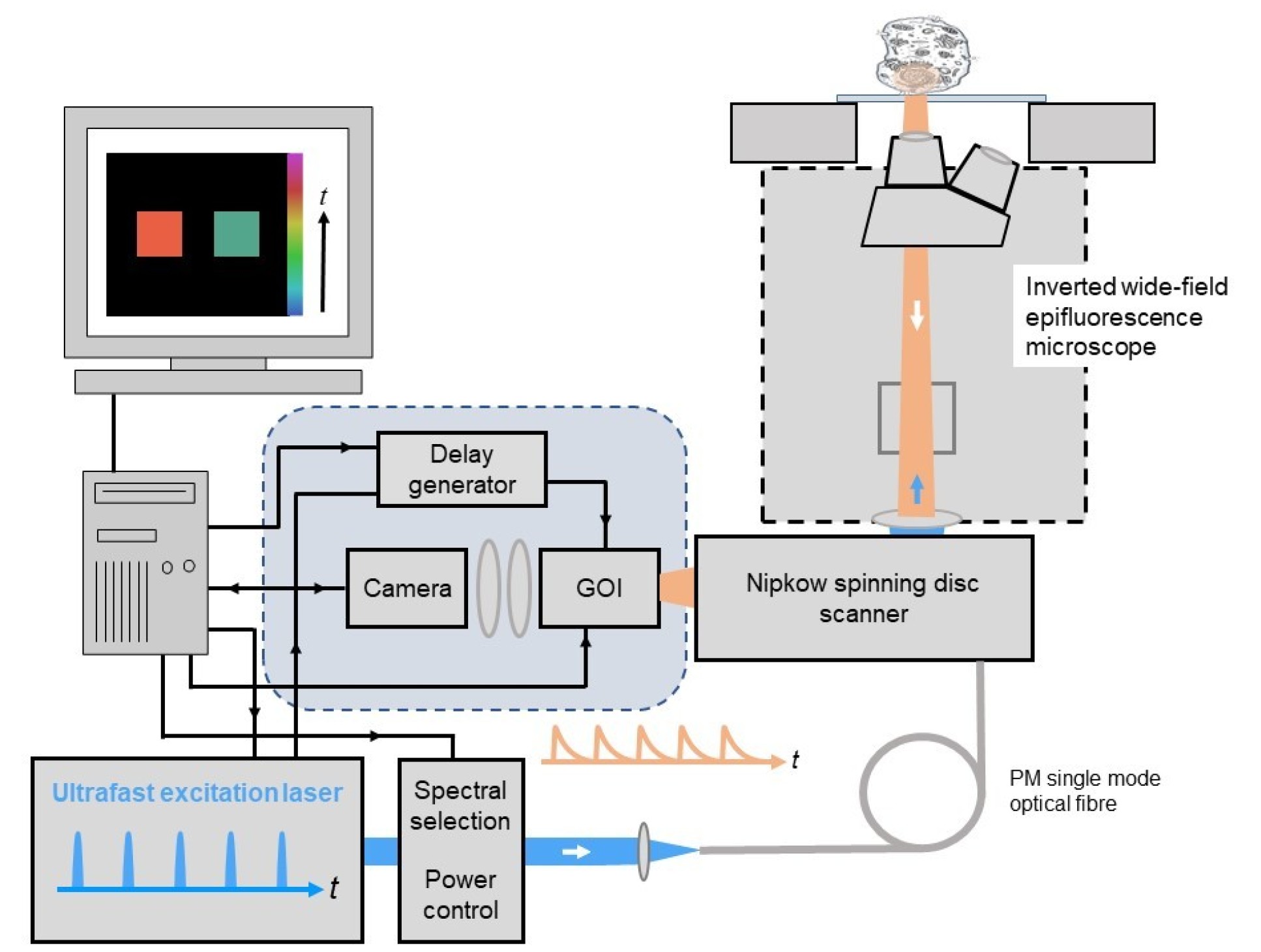
openFLIM hardware
For a list of components to assemble a wide-field time-gated FLIM microscope system or an optical sectioning wide-field FLIM micrscope utilising a Nipkow spining disc confocal scanner, please downlod openFLIM hardware.
openFLIM software
For FLIM data acquisition, we use our open source software, openFLIM-GOI, which is a MicroManager plug-in and runs on a standard personal computer requiring ~8 GB RAM with USB and serial ports to interface with the equipment components.
For FLIM data analysis, we use FLIMfit, our open source MATLAB-based software that provides a range of fitting techniques inclusing global analysis. This will run on a standard personal computer or laptop but we recommend investing in at least 64 GB RAM and a reasonable multicore processor if you intend to analyse large FLIM data sets. The FLIMfit software package is available as a client for the OMERO platform and can also be used as a stand-alone MATLAB application.
All our open source software for FLIM can be found here.
Applications of wide-field time-gated FLIM
These approaches to wide-field time-gated FLIM can be implemented on motorised microscopes with automated multiwell plate imaging capabilities for FLIM high content analysis.
Wide-field time-gated FLIM can also be combined with hyperspectral imaging to implement fluorescence lifetime resolved hyperspectral imaging and combined with polarisation-resolved imaging to realise time-resolved anisotropy imaging.
These FLIM approaches have been applied to study protein interactions in cell-based studies and to tissue autofluorescence to explore label-free diagnosis of disease, including cancer.
Exemplar publications utilisng wide-field time-gated FLIM
K. Dowling, M. Dayel, M. Lever, P. French, J. Hares, and A. Dymoke-Bradshaw, "Fluorescence lifetime imaging with picosecond resolution for biomedical applications," Opt. Lett. 23, 810-812 (1998); https://doi.org/10.1364/OL.23.000810
D. S. Elson, J. Siegel, S. E. D. Webb, S. Lévêque-Fort, M. J. Lever, P. M. W. French, K. Lauritsen, M. Wahl, R. Erdmann, “ Fluorescence lifetime system for microscopy and multi-well plate imaging using a blue picosecond diode laser”, Opt Lett, 27 (2002) 1409-1411; https://doi.org/10.1364/OL.27.001409
D. Grant, J. McGinty, E.J. McGhee, T.D. Bunney, D.M. Owen, C.B. Talbot, W. Zhang, S. Kumar, I. Munro, P. Lanigan, G. Kennedy, C. Dunsby, A.I. Magee, P. Courtney, M. Katan, M.A.A. Neil & P.M.W. French, “High speed optically sectioned fluorescence lifetime imaging permits study of live cell signaling events”, Opt. Expr.15 (2007) 15656 – 15673; https://doi.org/10.1364/OE.15.015656
D. M. Owen, E. Auksorius, H. B. Manning, C. B. Talbot, P. A.A. de Beule, M. A. A. Neil and P. M. W. French, “Excitation-resolved hyperspectral fluorescence lifetime imaging using a UV-extended supercontinuum source”, Opt. Lett. 32 (2007) 3408-3410; https://doi.org/10.1364/OL.32.003408
Richard K. P. Benninger, Bjorn Önfelt, Oliver Hofmann, Daniel M. Davis, Mark A. A. Neil, Paul M. W. French and Andrew J. DeMello, “Fluorescence‐Lifetime Imaging of DNA–Dye Interactions within Continuous‐Flow Microfluidic Systems”, Angewandte Chemie International Edition, 46 (2007) 2228-2231; https://doi.org/10.1002/anie.200604112
D. M. Grant, W. Zhang, E. J. McGhee, T. D. Bunney, C. B. Talbot, S. Kumar, I Munro, C. Dunsby, M. A. A. Neil, M. Katan and P. M. W. French, “Multiplexed FRET to monitor multiple signalling events in live cells”, Biophysical Journal: Biophysical Letters 95 (2008) L69-L71; https://doi.org/10.1529/biophysj.108.139204
J. McGinty, N. P Galletly, C. Dunsby, I. Munro, D. S Elson, J. Requejo-Isidro, P. Cohen, R. Ahmad, A Forsyth, A. V Thillainayagam, M. A. A. Neil, P. M W French and G.W Stamp, “Wide-field fluorescence lifetime imaging of cancer”, Biomedical Optics Express, 1 (2010) 627-640; https://doi.org/10.1364/BOE.1.000627
J. McGinty, H. Taylor, L. Chen, L. Bugeon, J. Lamb, M. Dallman, and P. French, "In vivo fluorescence lifetime optical projection tomography," Biomed. Opt. Express 2, 1340-1350 (2011) https://doi.org/10.1364/BOE.2.001340
D. Alibhai, D. J. Kelly, S. Warren, S. Kumar, A. Margineanu, R. A. Serwa, E. Thinon, Y. Alexandrov, E. J. Murray, F. Stuhmeier, E. W. Tate, M. A.A. Neil, C. Dunsby and P. M.W. French, “Automated fluorescence lifetime imaging plate reader and its application to Förster resonant energy transfer readout of Gag protein aggregation”, J. Biophotonics 6 (2012) 398-408, http://dx.doi.org/10.1002/jbio.201200185
D. J. Kelly, S. C. Warren, S. Kumar, J. L. Lagarto, B T. Dyer, A. Margineanu1, E. W.-F. Lam, C. Dunsby and P. M. W. French, “An automated multiwell plate reading FLIM microscope for live cell autofluorescence lifetime assays”, J. Innov. Opt. Health Sci.. 7 (2014) 1450025-15 pages, http://dx.doi.org/10.1142/S1793545814500254
Hugh Sparks, Sean Warren, Joana Guedes, Nagisa Yoshida , Nadia Guerra, Taran Tatla, Chris Dunsby, Paul French, “A flexible wide-field FLIM endoscope utilising blue excitation light for label-free contrast of tissue”, J Biophotonics 8 (2015) 168–178 / http://dx.doi.org/10.1002/jbio.201300203
D. J. Kelly, S. C. Warren, D. Alibhai, S. Kumar, Y. Alexandrov, I. Munro, A. Margineanu, J. McCormack, N. J. Welsh, R. A. Serwa, E. Thinon, M. Kongsema, J. McGinty, C. Talbot, E. J. Murray, F. Stuhmeier, M. A.A. Neil, E. W. Tate, V. M. M. Braga, E. W.-F. Lam, C. Dunsby and P. M.W. French, “Automated multiwell fluorescence lifetime imaging for Förster resonant energy transfer assays and High Content Analysis”, Analytical Methods, 7 (2015), 4071-4089, http://dx.doi.org/10.1039/C5AY00244C
N. Andrews, M-C Ramel, S. Kumar, Y. Alexandrov, D. J. Kelly, S. C. Warren, L. Kerry, N. Lockwood, A. Frolov, P. Frankel, L. Bugeon, J. McGinty, M. J. Dallman* and P. M. W. French*, “Visualising apoptosis in live zebrafish using fluorescence lifetime imaging with optical projection tomography to map FRET biosensor activity in space and time”, J. Biophoton, 9 (2016) 414–424. http://dx.doi.org/10.1002/jbio.201500258
F. Görlitz*, D. J. Kelly*, S. C. Warren, D. Alibhai, L. West, S. Kumar, Y. Alexandrov, I. Munro, J. McGinty, C. Talbot, R. A. Serwa, E. Thinon, V. da Paola, E. J. Murray, F. Stuhmeier, M. A. A. Neil, E. W. Tate, C. Dunsby and P. M. W. French, “Open Source High Content Analysis Utilizing Automated Fluorescence Lifetime Imaging Microscopy”, J. Vis. Exp. 119, (2017) e55119, http://dx.doi.org/10.3791/55119
H. Sparks*, F. Görlitz*, D.J. Kelly, S.C. Warren, P.A. Kellett, E. Garcia, A.K.L. Dymoke-Bradshaw, J.D. Hares, M. A.A. Neil, C. Dunsby, P.M.W. French, “Characterisation of new gated optical image intensifiers for fluorescence lifetime imaging”, Rev Sci Instrum. 88 (2017) 013707. http://dx.doi.org/10.1063/1.4973917
Frederik Görlitz, David S. Corcoran, Edwin A. Garcia Castano, Birgit Leitinger, Mark A. A. Neil, Christopher Dunsby and Paul M. W. French, “Mapping Molecular Function to Biological Nanostructure: Combining Structured Illumination Microscopy with Fluorescence Lifetime Imaging (SIM + FLIM)”, Photonics 4 (2017) 40; http://dx.doi.org/10.3390/photonics4030040
Wenjun Guo, Sunil Kumar, Frederik Görlitz, Edwin Garcia, Yuriy Alexandrov, Ian Munro, Douglas J. Kelly, Sean Warren, Peter Thorpe, Christopher Dunsby*, Paul French*, “Automated FLIM HCA of protein-protein interactions between endogenously-labelled kinetochore proteins in live budding yeast cells”, SLAS Technology, 24 (2019) 308-320; https://doi.org/10.1177/2472630318819240