Double detection of cell changes could diagnose disease earlier
Chemical probes that detect changes in two factors related to inflammation within cells could lead to earlier disease detection.
The probes measure changes in the levels of carbon monoxide (CO) and the viscosity (stickiness) of the fluid inside cells, two measures known to be associated with inflammation and are potential markers for heart disease.
They could be used when testing biopsy samples for signs of disease, and are also revealing the fundamental mechanisms of how disease affects cells, which could lead to new treatment approaches.
Probes capable of providing this degree of information on the cellular environment could be used to aid the early diagnosis of disease. Dr James Wilton-Ely
The probes were designed by researchers from the Department of Chemistry at Imperial College London. Their research is published in Angewandte Chemie, International Edition.
CO is naturally produced in the cells of mammals, where it has a range of roles, including helping to reduce inflammation. Therefore, when inflammation is present, levels of CO rise.
Another parameter that changes during inflammation is viscosity, or ‘stickiness’, of the fluid inside cells. Viscosity appears to change when levels of short-lived reactive chemicals called reactive oxygen species (ROS) change, which can affect signalling and transport of molecules within cells.
Measuring both CO and viscosity could therefore shed light on the mechanism of disease, such as whether ROS are involved, or even provide early disease diagnoses. However, creating probes that can pinpoint these changes within the complex environment of the cell has proven difficult.
Both parameters at once
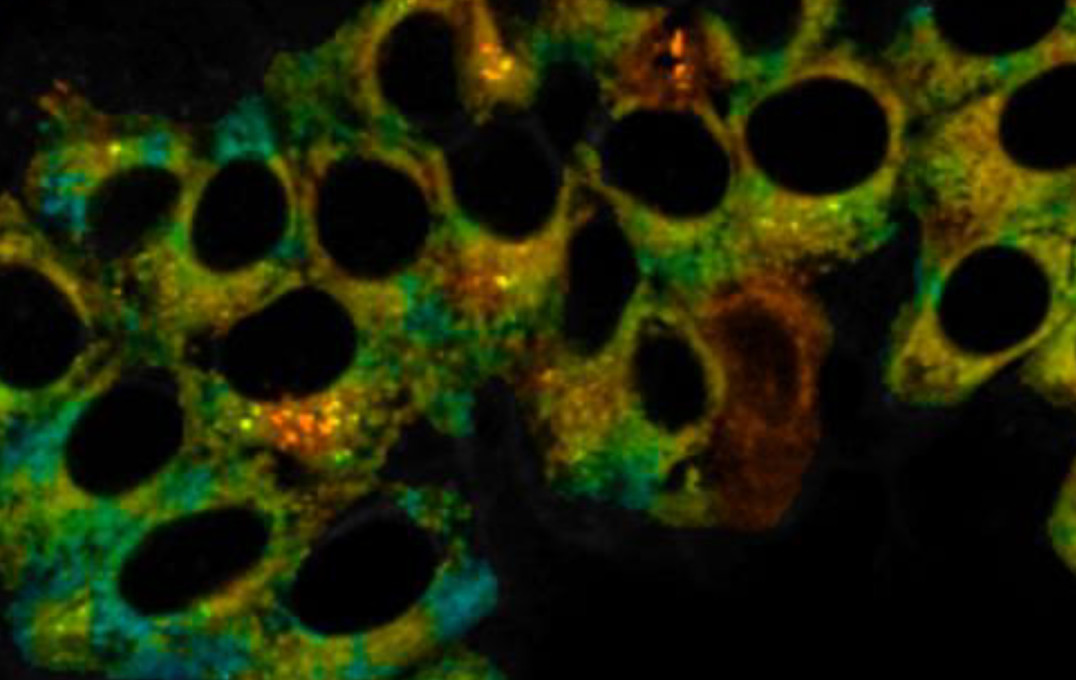
Now, the research team from Imperial have combined CO and viscosity sensing molecular probes and shown they can successfully measure the change in both parameters in cells in the lab.
Group leader, Dr James Wilton-Ely, from the Department of Chemistry at Imperial, said: “For the first time, we have created a probe that lets us see changes in both parameters at the same location in the cell. Probes capable of providing this degree of information on the cellular environment could be used to aid the early diagnosis of disease.
“Such tools also provide key information on the mechanisms used by our bodies to fight disease and could be used to design more effective interventions.”
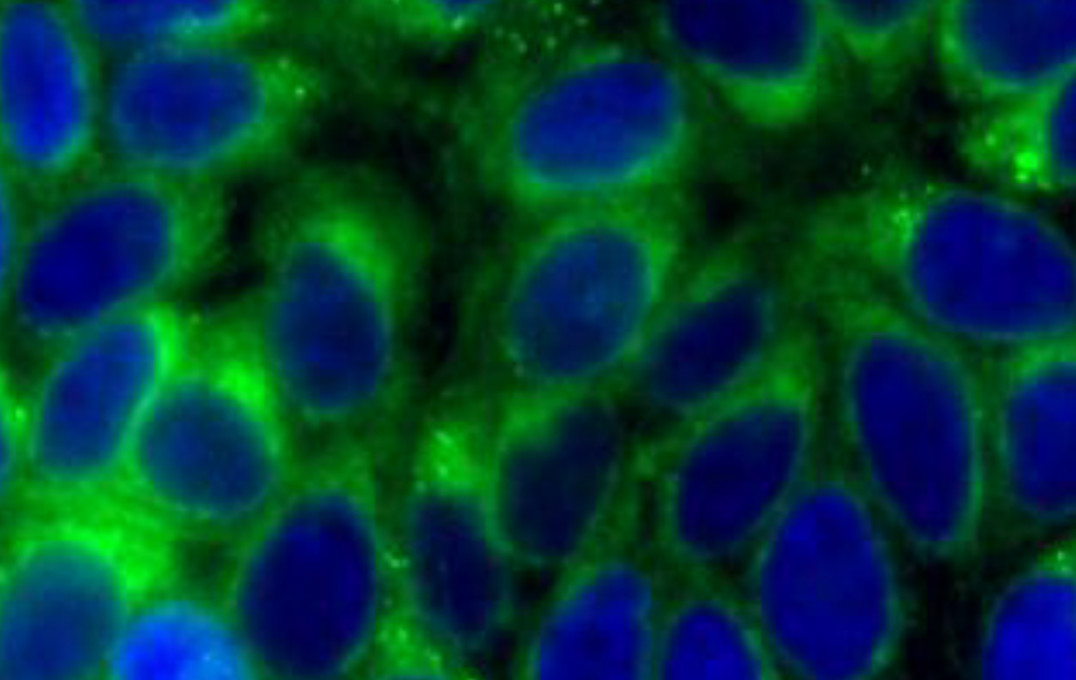
Dr Wilton-Ely and his team previously designed a probe to detect CO in cells taken from mice, using a molecule that fluoresces (glows) when the probe binds to CO so that the signal can be detected by microscope.
The team joined with another group in the Department of Chemistry, led by Dr Marina Kuimova, that has expertise in detecting viscosity changes using ‘molecular rotors’. The rate at which the fluorescence of these molecules decays over time depends on the internal flexibility within the molecule, which in turn is affected by the viscosity. Higher viscosity thus leads to slower rotation and results in a longer fluorescence lifetime.
Dual sensitivity
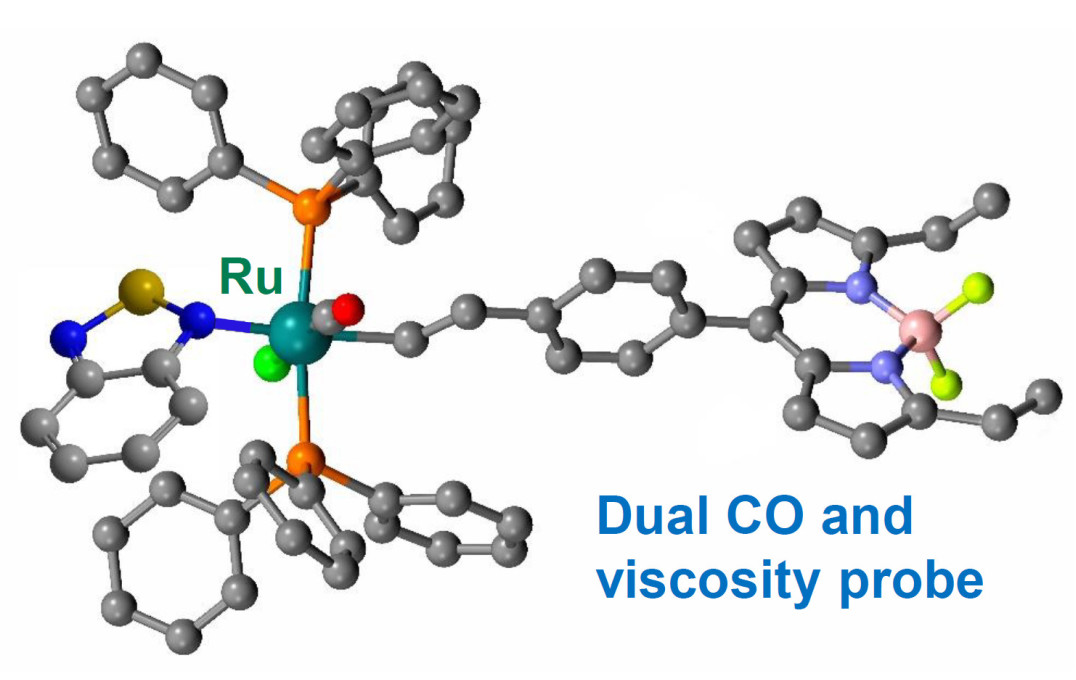
A shared office allowed members of the two teams – Dr Jonathan Robson and Dr Markéta Kubánková – to consider combining their probe designs. They created a probe with a ruthenium metal centre that binds selectively to CO and causes a 16-fold enhancement in the fluorescence. At the same time, a molecular rotor unit responds to viscosity by changing its fluorescence lifetime.
Dr Kuimova said: “The dual sensitivity of these new probes is unique and exploits the fact that different fluorescence parameters of the same probe (intensity and lifetime in this case) can be engineered to respond to independent stimuli.”
After confirming the probe was stable, non-toxic and was not affected by factors other than CO and viscosity, the team proved its detection ability in live cells. They ‘stressed’ the cells by creating an oxygen-deprived environment, which mirrors inflammation and causes the release of CO.
As well as detecting disease, the probes could be used to unravel other questions about cellular responses to inflammation. To this end, Dr Wilton-Ely and colleagues at King’s College London plan to explore the relationship between a cancer patient’s response to chemotherapy and their expression of an enzyme called HO-1, which generates carbon monoxide and is linked to suppression of the immune system.
-
‘Simultaneous Detection of Carbon Monoxide and Viscosity Changes in Cells’ by Jonathan A. Robson, Markéta Kubánková, Tamzin Bond, Rian A. Hendley, Andrew J. P. White, Marina, K. Kuimova and James D. E. T. Wilton-Ely is published in Angewandte Chemie, International Edition.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Hayley Dunning
Communications Division