Research shows how human cells are protected when the immune system switches on
Researchers show how a mechanism that human cells use to protect themselves against overzealous immune responses can be hijacked by invading bacteria.
The study, led by Imperial College London researchers, uncovers a way in which our immune system regulates itself, and how bacteria can exploit these processes. The research is published in Nature Communications.
Using state-of-the art imaging techniques we’ve been able to answer a long-standing question in the field of immunology. Dr Doryen Bubeck
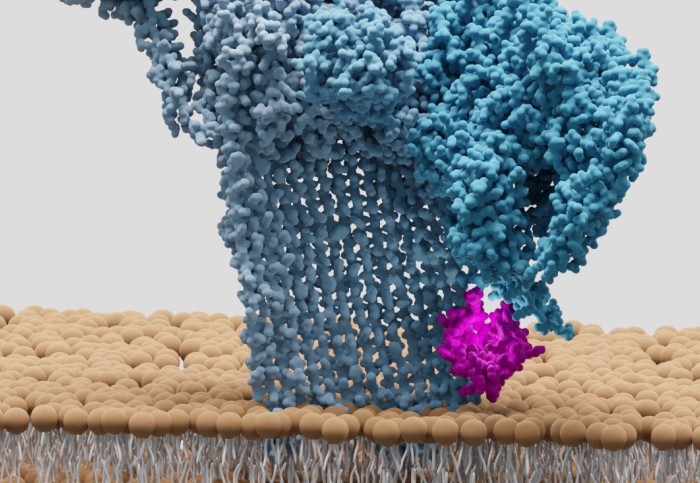
The team studied membrane attack complexes (MACs) – components of our immune system that punch holes in the membrane of invading bacteria. If enough holes are punched, the bacteria will burst and die.
Because MAC is such a powerful killing machine, human cells need a way to protect themselves from MAC. A small protein on the surface of human cells called CD59 is the body’s last line of defence against MAC. Without it, MAC can burst red blood cells when the immune system is turned on, causing human disease.
Researchers created an artificial membrane system that closely mimics a cell surface. Using this system, they were able to catch MAC as it forms and see in high molecular detail how CD59 stops MAC on the membrane.
Sparing human cells
Lead researcher Dr Doryen Bubeck, from the Department of Life Sciences at Imperial, said: “Using state-of-the-art imaging techniques we’ve been able to answer a long-standing question in the field of immunology. We’ve discovered how human cells are spared when MAC is deployed by the immune system to fight pathogens.”
The team used cryo-electron microscopy (cryoEM) to image in detail forms of MAC that had been inhibited by CD59 on artificial membranes. They discovered how CD59 captures and redirects parts of the MAC that aim to breach the cell’s membrane.
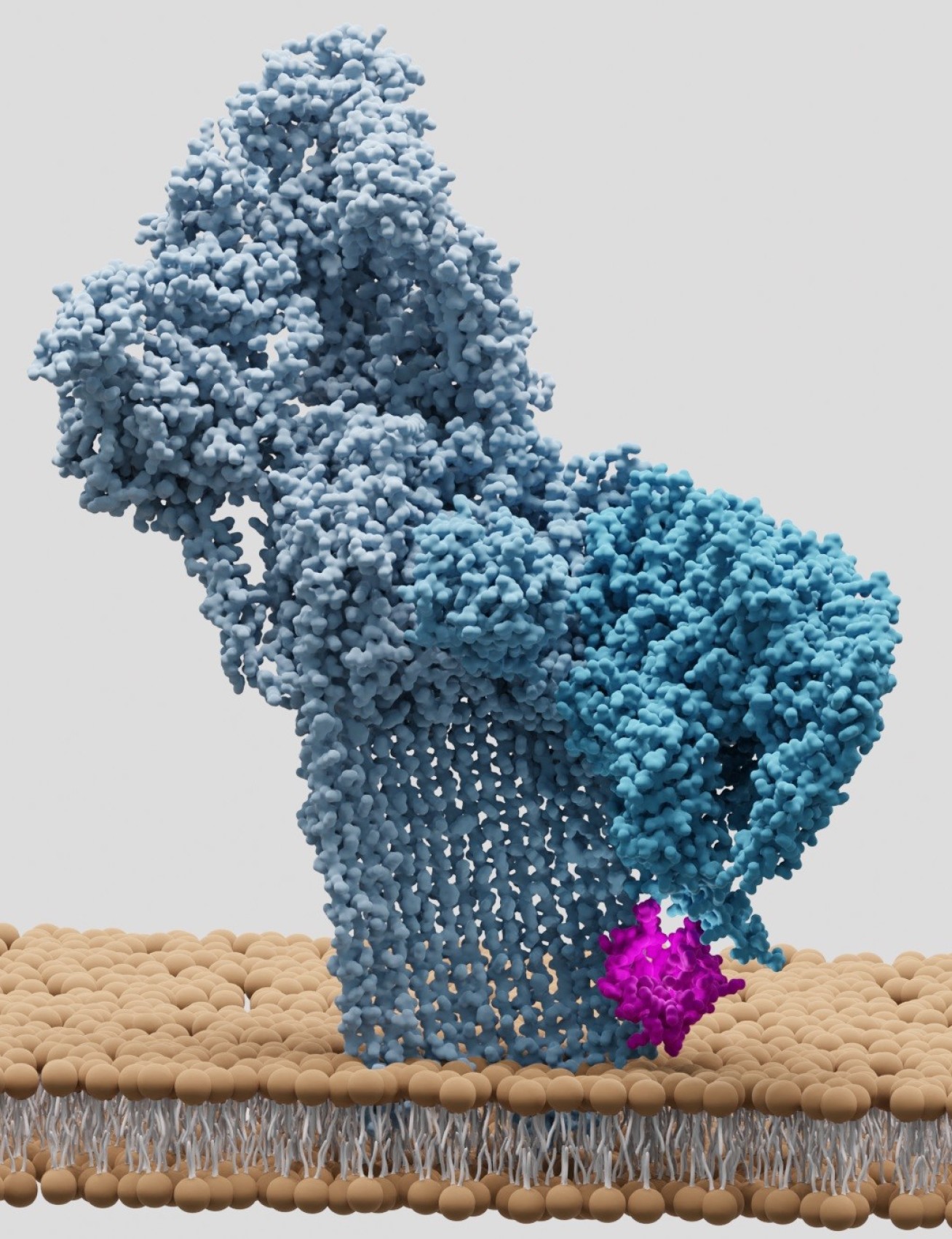
This not only blocks the pore from forming, but the team also found CD59 can stop the MAC itself forming properly – stopping the assembly of a structure over 100 times its own size.
However, there is a downside to CD59, as it can also be hijacked by bacterial proteins to target human cells for invasion. Previous work by the team published in Cell Reports showed that the parts of CD59 that interact with bacterial proteins are the same parts responsible for stopping MAC.
The big difference is the orientation of CD59 on the cell surface. The team’s new investigation showed how this is possible using computer modelling which showed that CD59 is very mobile on the membrane. Bacterial proteins exploit this flexibility to co-opt CD59 for infection.
First author Dr Emma Couves said: "By solving multiple different cryoEM structures we were able to capture the complete inhibitory mechanism of CD59, explaining not only how the complement system is regulated by the host but also how it is hijacked by tumours and pathogens."
Disease insights
These insights help researchers understand how an immune response is regulated to prevent inflammation and damage to human cells. It also has broader implications for how we might be able to tune this process during cancer immunotherapies that work by activating the complement immune system, of which MACs are a part.
Discovering more about how host cells and pathogens interact could also have important implications for understanding mechanisms of infectious diseases, and how to tackle them.
This work is part of ongoing work in the lab funded by a European Research Commission Consolidator Grant to investigate complement immune system control mechanisms.
-
‘Structural basis for membrane attack complex inhibition by CD59’ by Emma C. Couves, Scott Gardner, Tomas B. Voisin, Jasmine K. Bickel, Phillip J. Stansfeld, Edward W. Tate and Doryen Bubeck is published in Nature Communications.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Hayley Dunning
Communications Division