New research advances understanding of Carbon Capture and Storage technology
by Gemma Ralton

Scientists conducted important research into the solubility of hydrogen to aid the development of Carbon Capture and Storage technology.
As countries across the globe transition towards net zero emissions, the investment in new technologies such as Carbon Capture and Storage (CCS) and the use of hydrogen (H2) as an alternative fuel gas is becoming critical.
In a recent study from Imperial College London, published in Fluid Phase Equilibria, scientists conducted important research into the solubility of hydrogen impurities in reservoir brines in an effort to enhance the future development and success of CCS systems. This involved studying hydrogen as it dissolved in a sodium chloride solution at varying pressures and temperatures.
Hydrogen impurities and solubility
Due to the technological limitations of existing energy networks, the current favoured method of hydrogen production involves natural-gas reforming coupled with CCS to form so-called ‘blue’ hydrogen. In this process, natural gas is reacted with steam to form hydrogen and carbon dioxide (CO2). The CO2 is then pumped to secure underground storage in depleted oil and gas reservoirs, coalbeds, or deep saline aquifers.
"From an industrial perspective, the research contributes to de-risking carbon dioxide storage by building a detailed scientific case to underpin predictions of the storage capacity and security of reservoirs.” Professor Martin Trusler Department of Chemical Engineering
Captured carbon dioxide is never pure, and the stream from blue hydrogen production will contain significant impurities of hydrogen. Establishing the extent and rate at which these hydrogen impurities dissolve in saline waters is important for predicting the long-term performance of a storage reservoir.
The recent study from Imperial addresses these knowledge gaps and was able to quantify for the first time the effects of salts on reducing solubility of hydrogen and other gases in brines under conditions that replicated those in deep saline aquifers.
The work was conducted as part of the recently-completed ELEGANCY project, an international collaborative effort funded under the Accelerating CCS Technologies Programme with the aim of fast-tracking the decarbonisation of Europe’s energy system through hydrogen and CCS.
Carbon Capture and Storage capacity
Carbon Capture and Storage is the method of capturing carbon dioxide formed during power generation and industrial processes and storing it so that it is not emitted into the atmosphere.
The technology has significant potential to capture almost all of the CO2 produced by these processes, with the greatest storage capacity offered by deep saline aquifers.
In order to meet existing net-zero targets, estimates suggest that the deployment of CCS technology must increase by several orders of magnitude within the next two decades.
However, a number of barriers must be overcome in order to accelerate widespread CCS deployment. One such barrier involves de-risking the storage process by improving our fundamental understanding of the long-term fate of the injected CO2.
Blue hydrogen for a green future
In many decarbonisation scenarios, ‘blue’ hydrogen is considered to be a promising renewable energy carrier with uses in fuel, heating, transport and industry. Yet despite its advantages, there are still issues to address.
Blue hydrogen refers to the splitting of natural gas into hydrogen and CO2 by either Steam Methane Reforming or Auto Thermal Reforming. The carbon dioxide released in the process is captured and stored to mitigate environmental impacts.
Hydrogen can also be produced by splitting water through electrolysis to form only oxygen and hydrogen, otherwise known as ‘green’ hydrogen. In this process, the electricity is generated from renewable sources such as wind and solar with near-zero CO2 emissions.
Due to the practical limitations of current energy systems, it is unlikely that green hydrogen can be rolled out at scale before 2050. Blue hydrogen with CCS therefore offers the optimal solution at present.
However, there are still several issues to be addressed particularly concerning the solubility of hydrogen impurities in storage reservoirs. Prior to the current project, there were no high-pressure data of hydrogen solubility in saline water. This is due to the complexity of this type of measurement, characterised by high pressure, high flammability of hydrogen and presence of salt which therefore risks corrosion and leakage.
Similarly, reliable knowledge of the thermophysical properties of mixtures of CO2, H2 and other substances are essential in designing the overall process of hydrogen production from fossil fuels with simultaneous capture, transportation, and storage of CO2.
Pioneering technology
The recent study conducted by scientists at Imperial is the first of its kind to address these issues for hydrogen, using a new experimental apparatus which implemented a static synthetic method designed specifically for this work.
The novel apparatus was built to measure precisely the solubility of hydrogen in water or brine under controlled temperature and pressure conditions. The equipment can withstand up to 150°C and a pressure of up to approximately 400 bar to mimic the conditions found in naturally occurring deep saline aquifers commonly used in CCS.
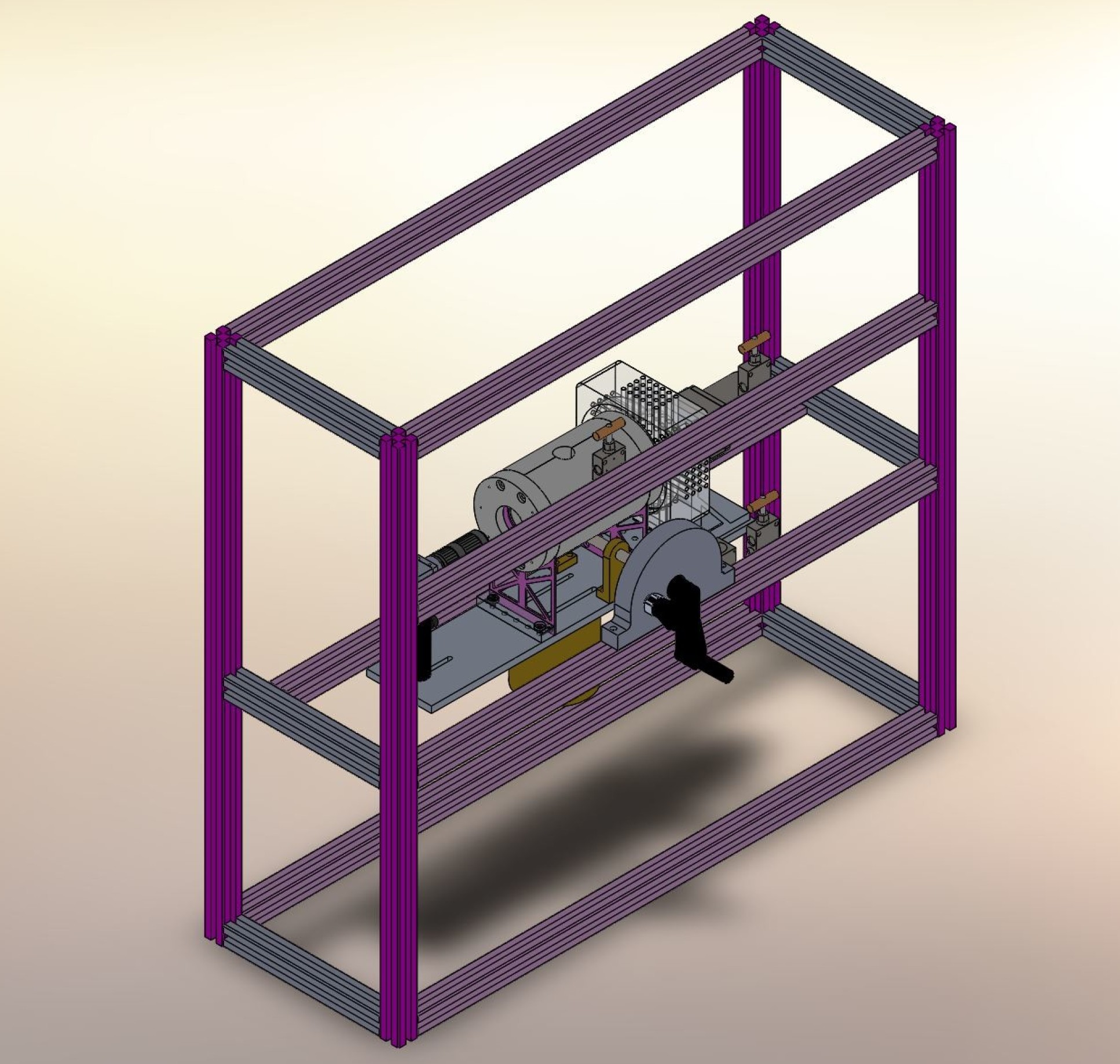
Using this method, a known amount of gas was first introduced into a high pressure vessel. Next, water or brine was injected, compressing the gas and forcing some of it into solution, and this was continued until all the gas had dissolved.
The process was monitored by visual observation through a sealed window, made from a single crystal of pure sapphire, which formed one end of the vessel.
The experiment was repeated at different temperatures and with varying amounts of gas to establish how gas solubility varies against temperature and pressure.
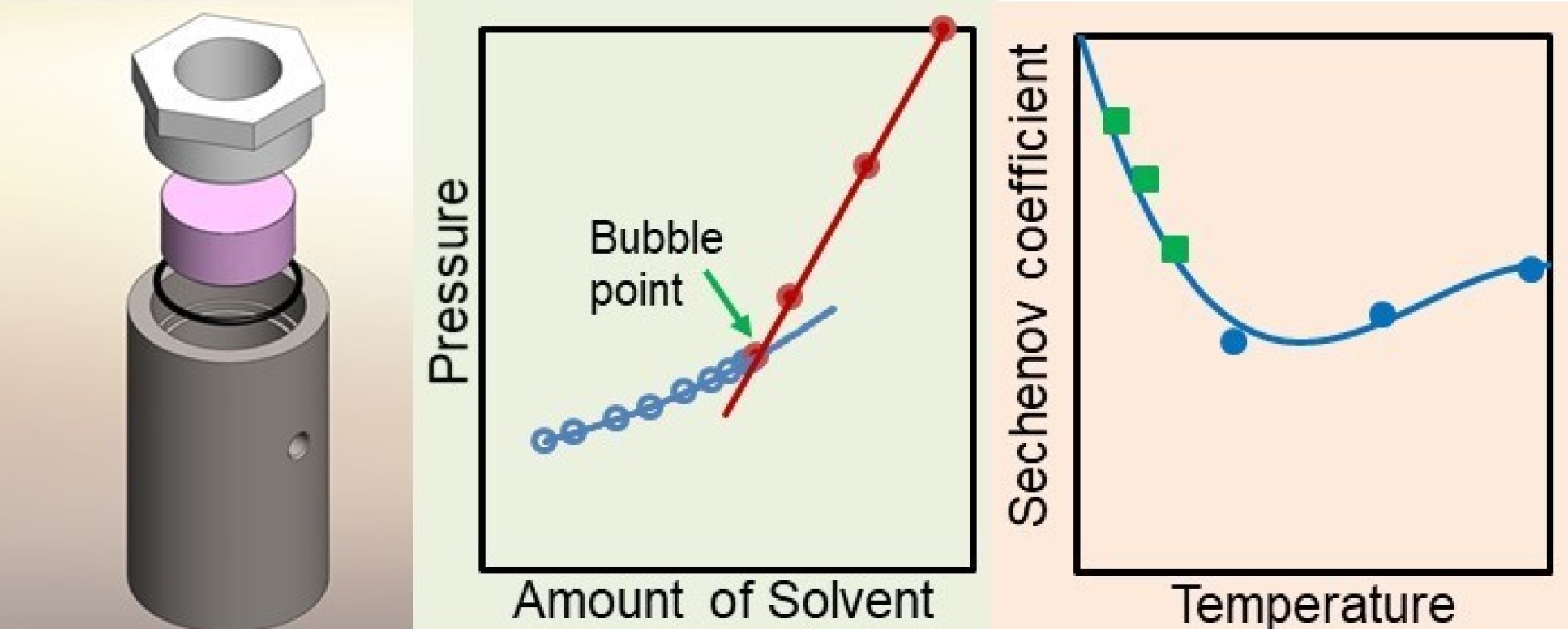
An important feature of the design, based on lessons previously learned, was the elimination of any unobservable ‘dead’ volumes in which undissolved gas could be trapped.
The design also featured materials, such as titanium alloy and sapphire, that are resistant to corrosion by concentrated brines.
According to Principle Investigator Professor Martin Trusler: “From an industrial perspective, the research contributes to de-risking carbon dioxide storage by building a detailed scientific case to underpin predictions of the storage capacity and security of reservoirs.”
In other words, the study will help to better predict the long-term performance of Carbon Capture and Storage facilities to ensure increasing future success of the technology."
Moving forward
The uptake of Carbon Capture and Storage technologies is currently slow, limited by lack of financial incentives, low public acceptance, lack of consistent government energy policy and technological limitations.
The research conducted in this study in conjunction with the ELEGANCY project represents a critical step towards aiding the widespread implementation of CCS technology and higher uptake of hydrogen as a fuel for a greener energy future.
Researchers suggest further investigation into other types of brine as their study covered solubility in sodium chloride solutions only. Moreover, discrepancies between competing studies with varying results must also be addressed moving forward.
-
‘Solubility of hydrogen in sodium chloride brine at high pressures’ by Torin-Ollarves and Trusler, published 18th March 2021 in Fluid Phase Equilibria.
Main image: Shutterstock by Roman Zaiets.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Gemma Ralton
Faculty of Engineering