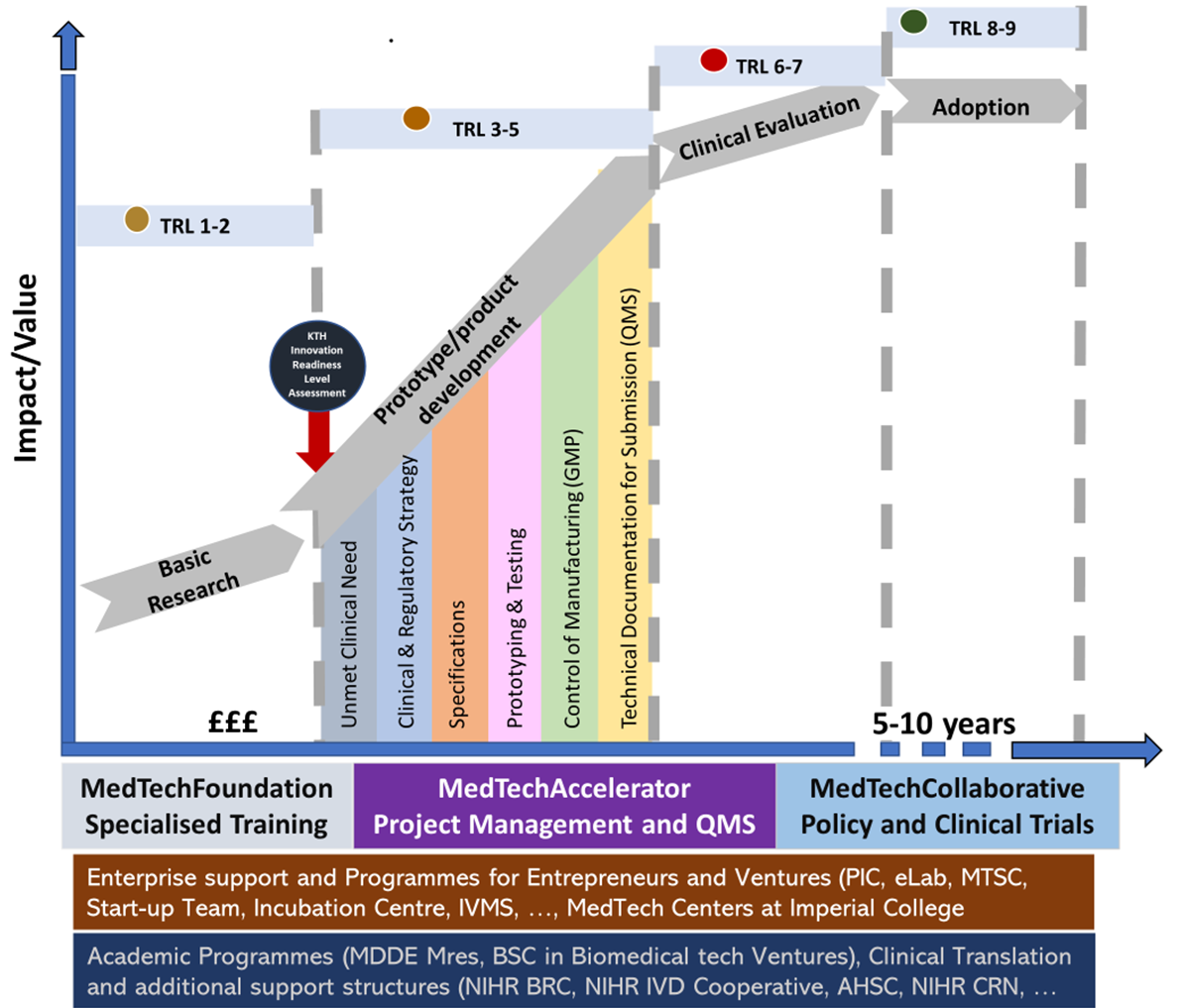
Quality Assurance and Regulatory Affairs (QARA) support is a central pillar of the MedTech Accelerator, ensuring that Imperial’s medical technology innovations are developed safely, systematically, and in alignment with UK, EU, and US regulatory expectations.
Medical device development is heavily regulated, and early compliance significantly reduces the risk of delays, redesign, or failed submissions later in the translation pathway. Our QARA function equips teams with the expertise, systems, and guidance needed to navigate this complexity with confidence.
Regulatory Pathway Guidance from the Start
Every MedTech project must meet regulatory requirements tailored to its risk classification, intended use, and clinical pathway. We help teams:
-
determine medical device classification
-
understand MHRA, FDA, and CE marking requirements
-
identify applicable standards (e.g., ISO 13485, ISO 14971, IEC 62366)
-
plan early design controls and verification strategies
-
map regulatory milestones to TRL and development stages
This ensures regulatory considerations inform technical decisions from the outset—not at the end of development when changes are costly.
Access to an ISO 13485-aligned Quality Management System (QMS)
The MedTech Accelerator provides teams with access to our embedded ISO 13485-aligned Quality Management System, enabling:
-
structured documentation practices
-
design and development planning
-
risk management according to ISO 14971
-
usability engineering under IEC 62366
-
change control and traceability
-
preparation of technical documentation for submission
Using a QMS early ensures teams generate the right evidence, avoid compliance gaps, and build strong foundations for regulatory approval.
Regulatory Readiness Level (RRL) Framework
To provide tailored support, we use the Regulatory Readiness Level (RRL) framework developed by MedTechONE.
This tool assesses a project’s regulatory maturity, identifies gaps, and guides teams through:
-
early regulatory strategy formulation
-
evidence planning
-
documentation generation
-
quality process implementation
-
readiness for clinical investigation
Support is adapted to the project’s TRL and RRL, ensuring the right expertise at each stage.
Practical Support for Key Regulatory Activities
Our QARA specialists provide hands-on guidance in:
-
defining intended purpose and indications for use
-
mapping essential performance and safety requirements
-
developing regulatory documentation (GSPR/ESR, design files, technical files)
-
preparing for MHRA, FDA, or Notified Body engagement
-
planning clinical evaluation and post-market requirements
-
integrating risk management throughout development
This reduces uncertainty and equips teams with the knowledge needed to progress toward approval or clinical investigation.
Enabling Safe and Compliant Innovation
Through close collaboration with the Translational Project Management function, IPC, the NIHR London IVD Cooperative, and the NIHR BRC Bioengineering Theme, QARA support ensures:
-
regulatory strategy informs project planning
-
quality processes align with commercialisation goals
-
teams are prepared for investor and industry scrutiny
-
documentation is robust enough for future submissions
By embedding regulatory and quality expertise early, we help teams avoid common pitfalls and position their innovations for successful adoption, licensing, or spinout.