Translational Project Management is a core function of the MedTech Accelerator, supporting teams to progress their medical technologies through structured, TRL-aligned development pathways.
MedTech innovations face interconnected technical, regulatory, clinical, and commercial challenges. TPM provides the coordinated, strategic support required to navigate this complexity and drive projects toward clinical validation and adoption.
A Structured Approach to MedTech Development
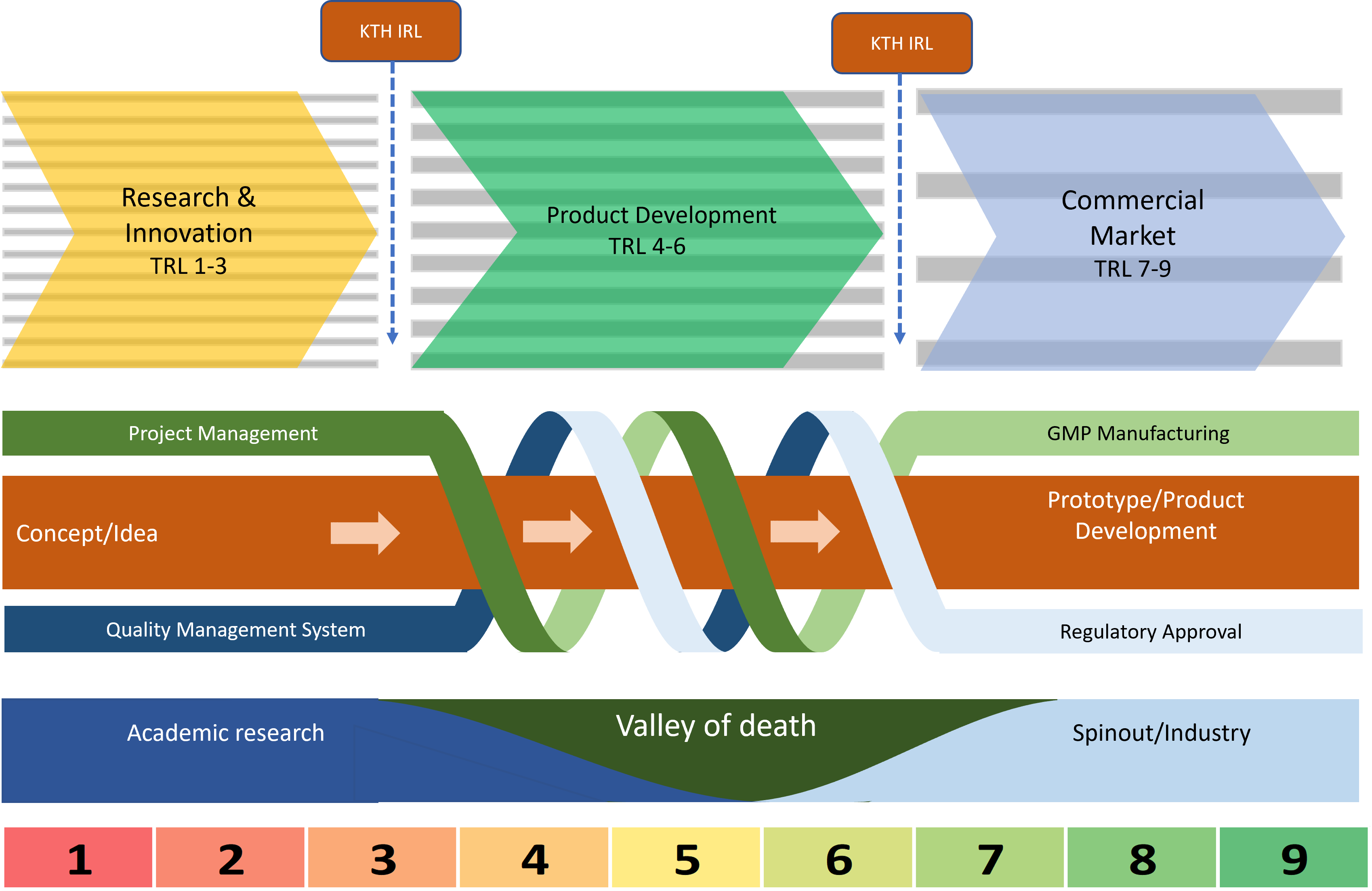
Every selected project undergoes a two-week Innovation Readiness Level (IRL) assessment using the KTH framework. This evaluation identifies gaps across technology, customer understanding, regulatory readiness, funding strategy, IP, and team capability.
From this, we co-develop a Development Action Plan that:
-
defines TRL-aligned milestones
-
clarifies technical, clinical, and regulatory requirements
-
strengthens the project’s value proposition
-
identifies critical risks and dependencies
-
aligns the development route with market and NHS needs
-
supports planning for follow-on funding, investment, and commercialisation
The TPM function ensures that each project follows a realistic and evidence-based translation pathway.
Guiding Projects Through the Translation Journey
Our project managers work closely with teams throughout the programme to:
-
coordinate technical, regulatory, clinical, and commercial activities
-
track progress using the MedTechONE Readiness Scoring system
-
maintain detailed development logs through the Project Passport
-
prepare teams for engagement with IPC, investors, and partners
-
connect teams with relevant internal and external expertise
-
support preparation for clinical evaluation and NHS engagement
This structured oversight keeps teams focused, accountable, and progressing efficiently.
Tailored Support Based on Project Maturity
We adapt support according to each project’s Technology Readiness Level (TRL) and Regulatory Readiness Level (RRL):
Early-stage projects (TRL 1–3)
-
validating unmet clinical need
-
early prototyping strategy
-
defining regulatory classification and design controls
-
customer and market exploration
Mid-stage projects (TRL 3–6)
-
prototype refinement and verification
-
regulatory pathway mapping
-
early quality processes
-
user engagement and feasibility planning
-
preparation for clinical studies
Later-stage projects (TRL 6–7)
-
documentation for regulatory submission
-
clinical investigation planning
-
manufacturing pathway preparation
-
investment readiness and commercial positioning
TPM ensures the right level of support is provided at the right time.
Integrated Support Across Imperial
TPM collaborates closely with:
-
Industry Partnerships & Commercialisation (IPC) – IP, licensing, commercial strategy
-
MedTech SuperConnector – entrepreneurship and venture development
-
NIHR BRC and NIHR London IVD Cooperative – clinical and diagnostic input
-
MedTechONE Foundation & Collaborative streams – training and clinical adoption support
This integrated ecosystem ensures that projects are strategically guided from concept toward clinical impact.
Driving Real Impacts
Imperial’s Translational Project Management model has already supported projects that have:
-
progressed to clinical evaluation
-
secured translational and commercial funding
-
formed spinouts
-
engaged with NHS partners
-
strengthened regulatory and commercial readiness