Introduction
A key aspect of the translation of medical devices is the pathway to getting a device into usage at hospitals. This process can be quite opaque, and isn’t always widely known. We’ve used the government policy guidance to create this resource, to give you a guide into the processes behind the scenes of medical device acquisitions in the NHS.
This webpage is a brief look into this resource. For the full information, please download the PDF documents available at the end.
Disclaimer: This information has been gathered from UK government guidance (August 2025). As such, this information may be subject to change. You can download a copy of the guidance we based this resource on at the end of the page.
What are Medical Devices?
The term "medical device" can encapsulate many types of device:
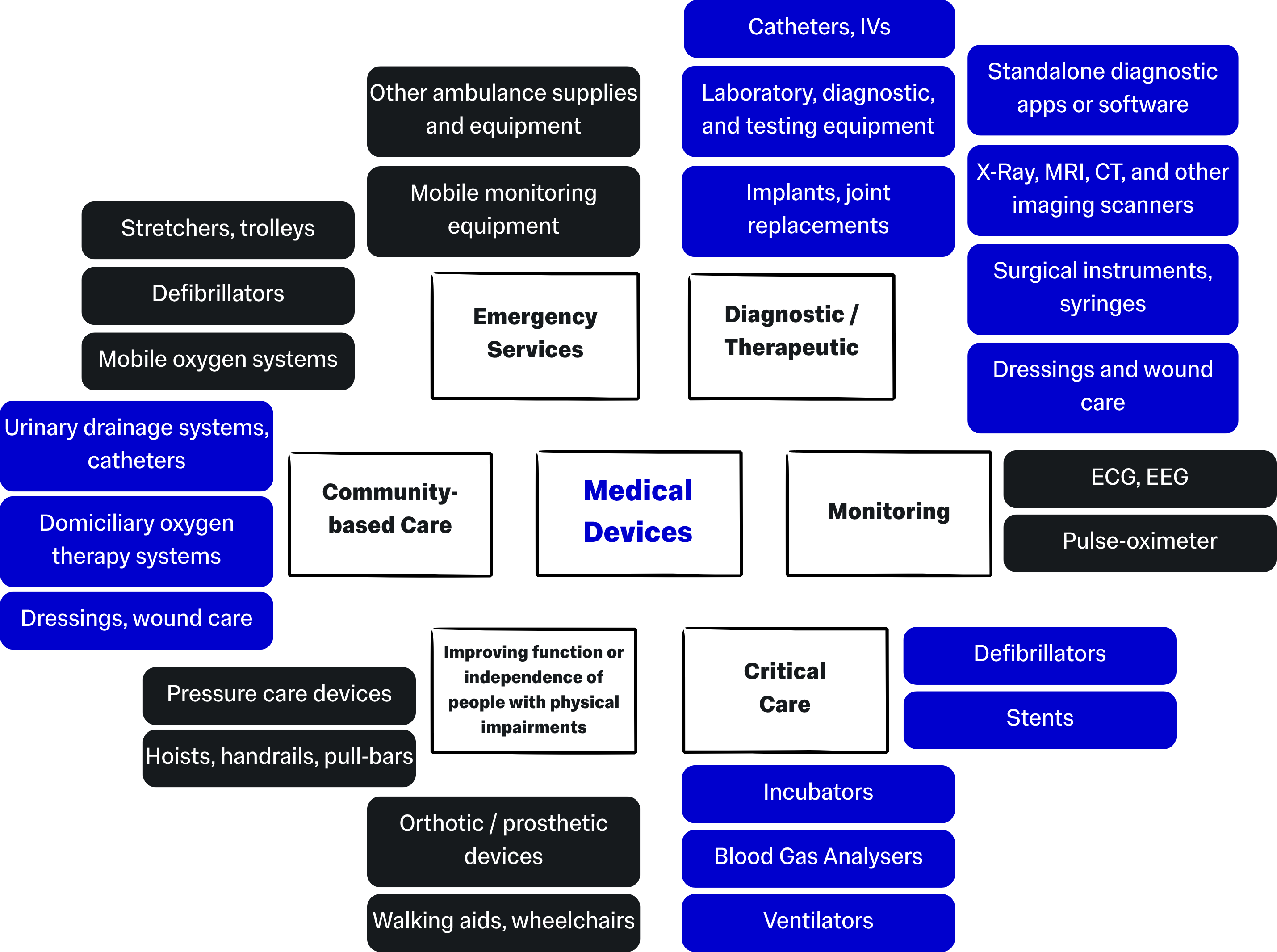
This guidance also covers In Vitro Diagnostic (IVD) devices, defined as:

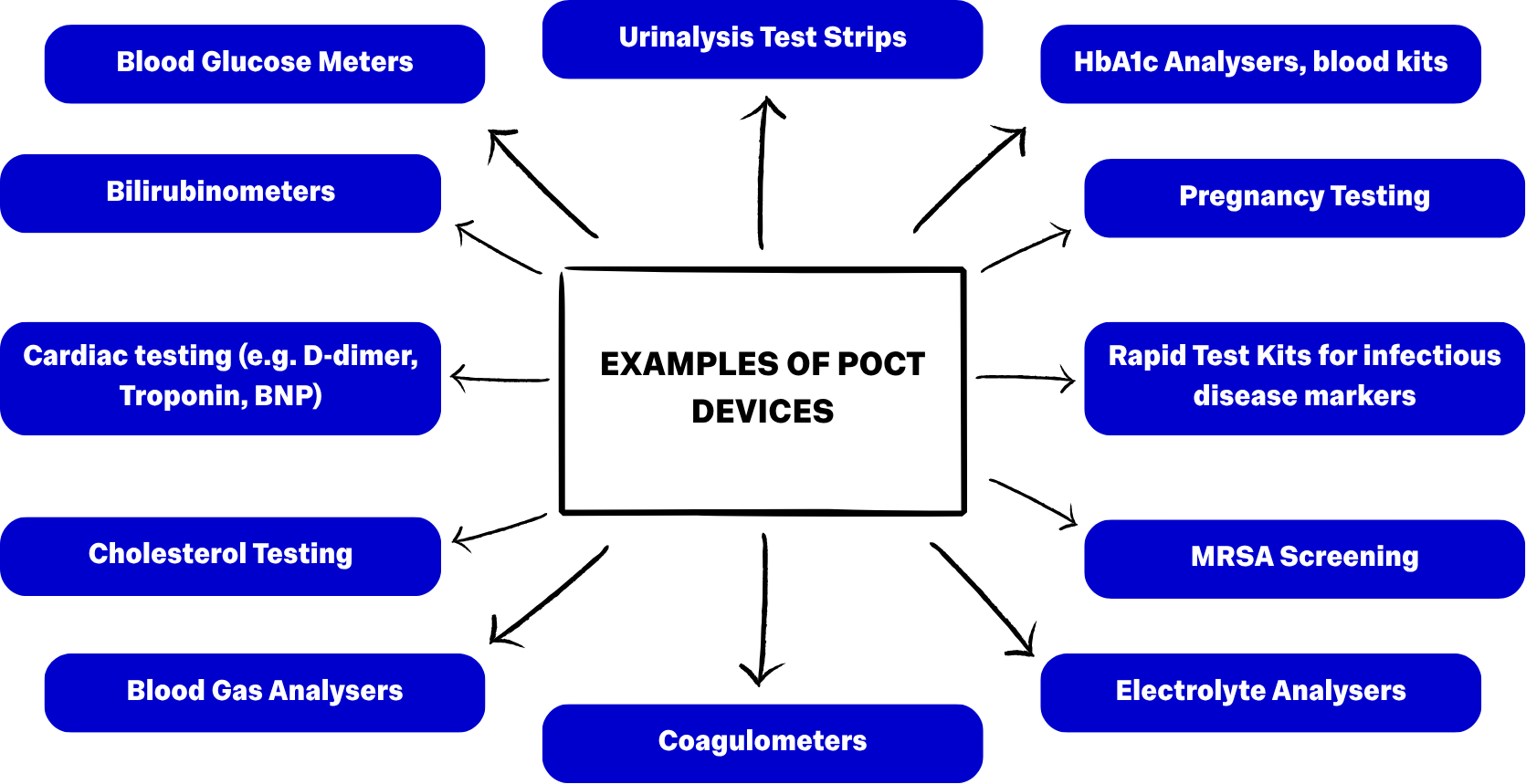
And Point of Care Testing Devices:
Understanding Hospital Structures
In the UK, hospitals will have set structures and hierarchies for all the people involved in the decision-making process for acquiring a new medical device or system.
As the manufacturer or supplier of a device, you will primarily be in contact with the Medical Device Management Group for that institution. Policies implemented by the MDMG will directly assess if a device is:
- Suitable for the intended purpose.
- Used in line with manufacturer instructions.
- Traceable (where possible).
- Maintained in a safe and reliable condition, with associated records maintained.
- Disposed of appropriately at the end of the life cycle, in accordance with Trust policy.
Who is responsible for the device?
Systems for managing medical devices will differ depending on the type of deployment that will apply to the device. Devices can be:
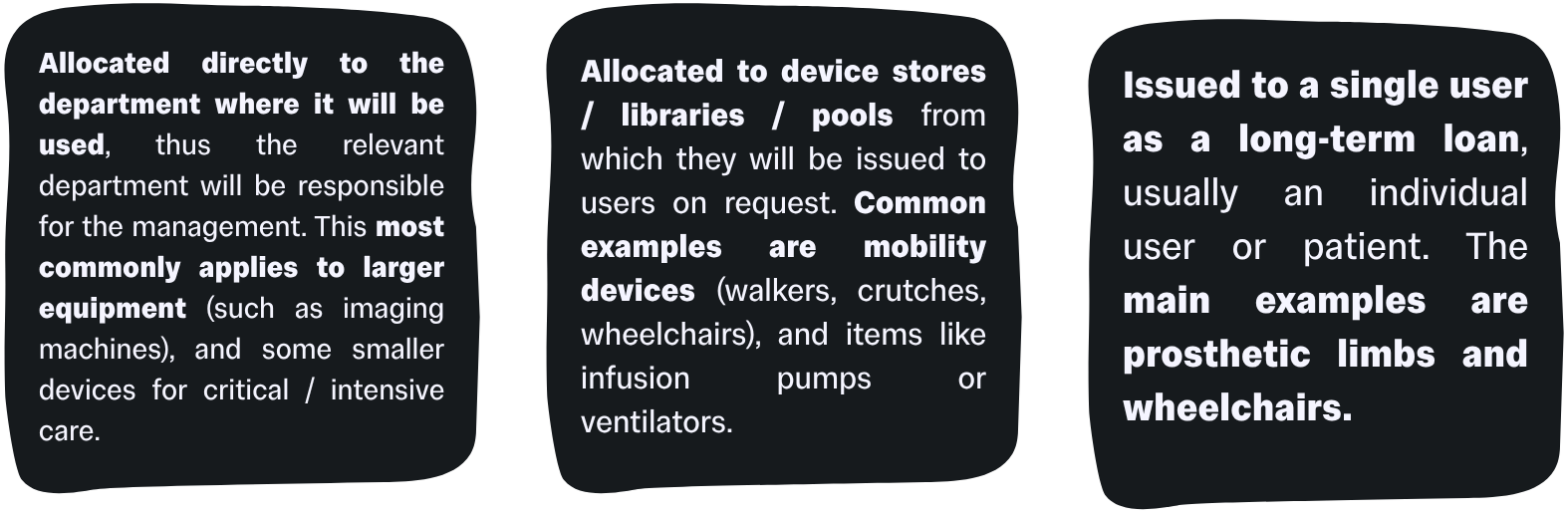
In community care-based settings, the devices’ management, maintenance and care will either be the responsibility of the end-user / patient, or the responsibility of a community worker.
For device libraries / pools, the device management and maintenance usually fall under the store’s purview.
For devices issued on long-term loans, the device management will usually be split between the end-user (day-to-day management and maintenance) and a healthcare provider (scheduled maintenance, end of life cycle disposal, major repairs etc).
Acquisition & Selection of Devices

Every healthcare organisation will have a policy in place for the selection and acquisition of medical devices, that usually includes a multi-year investment and replacement plan. It may include a short-to-long term schedule of medical devices, monitored against appropriate risk criteria.
Local acquisition policy will address:
- The clinical needs being addressed, and that the selection process considers care objectives, needs of patients, and the organisation’s healthcare priorities.
- Safety, quality and performance, as well as the acquisition cycle as a whole.
- Whole life costs associated with the device, in line with national acquisition policy, guidance and recommendations. Considerations to value for money.
- Considerations for the needs and reasonable preferences of all involved parties, which includes both those involved in the use of the device, but also those involved in the general maintenance, upkeep, decontamination, and decommissioning of the device.
- Cost effectiveness of consumables relating to the device – includes the base cost of the device, and the lifetime cost of all related consumables.
- The mechanisms for the selection and acquisition of medical devices for specific procedures.
N.B. healthcare organisations can be held legally responsible for any injury or death caused by inappropriate purchase, prescription or use of a device.
Methods of Acquisition
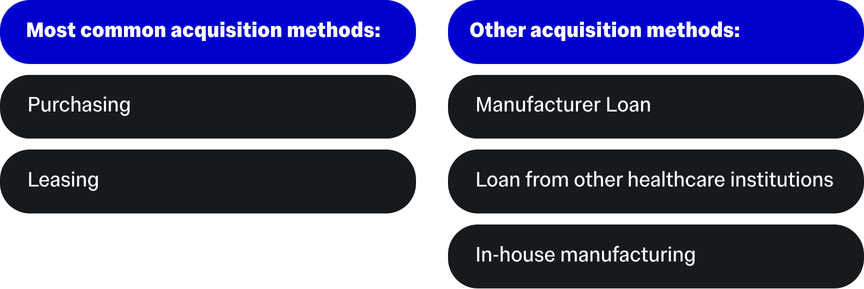
As an initial step, it's a good idea to consider how your medical device will fit into the hospital's existing ecosystem.
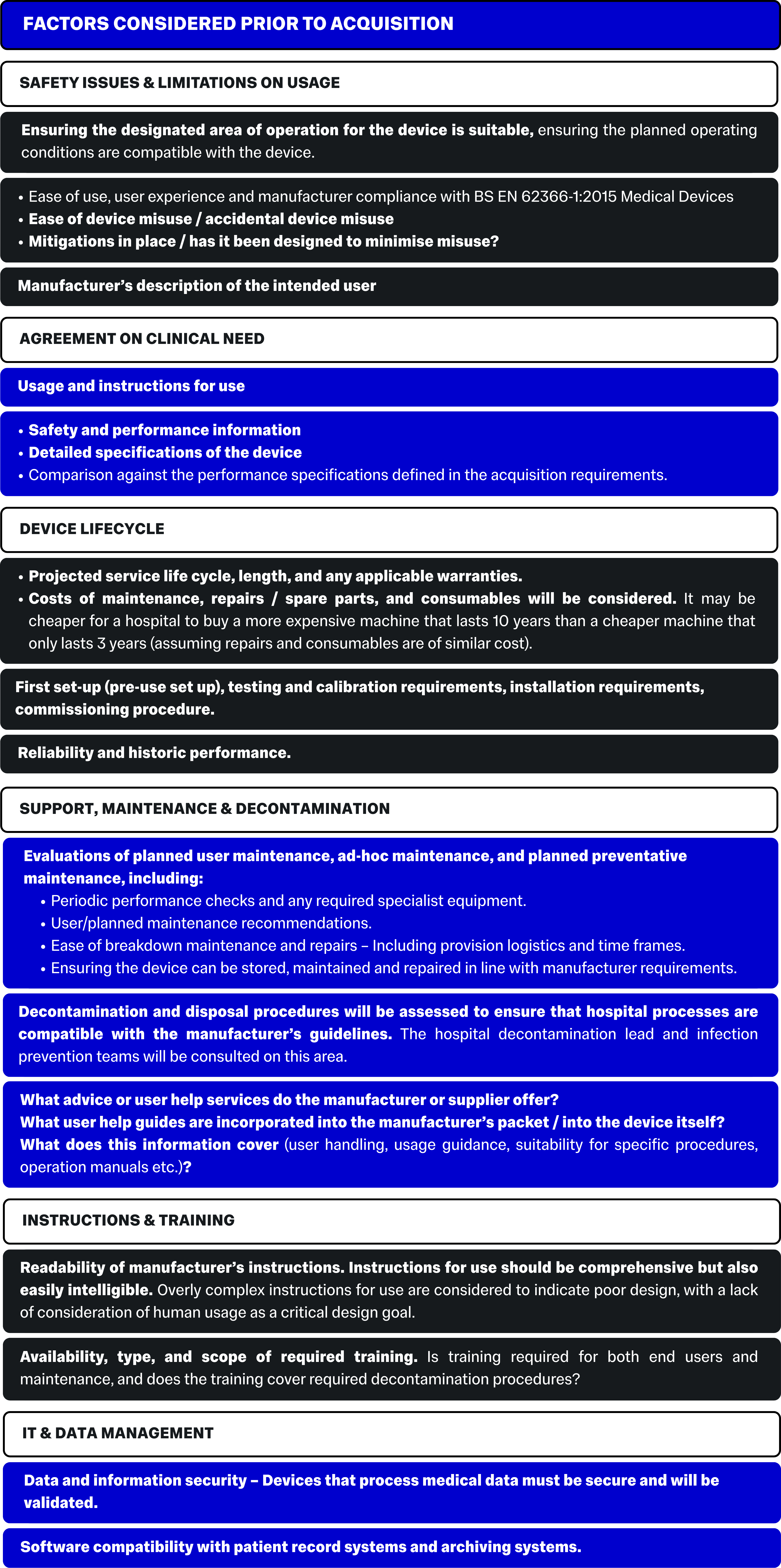
Safety Performance & Reliability

It is a legal requirement for all medical devices placed on the UK market to be UKCA / CE marked.
The hospital will also assess any local history of issues with the device or similar devices, and will consider any issues that the device may face when in situ.
Part of these checks will cover MHRA safety publications, manufacturer advisory notices and relevant publications. The hospital may also conduct a local level investigation into user experiences with the device, or similar devices, encompassing any known problems or failures.
The manufacturer may be asked to provide evidence of reliability from other responsible organisations and will need to supply evidence of compliance with relevant regulatory standards.
Rationalising the range of models vs diversity of models
Hospitals consider the risk of “operator confusion” when acquiring devices. This means that hospitals are unlikely to stock a variety of models for the same purpose/procedure.
It is however, understood that there are risks to having only one model:
- A chosen model may be unreliable
- A single model will eventually go out of production/warranty/be unavailable for repairs/may be withdrawn by the manufacturer etc.
The main concern is models/devices that are superficially similar but that have differing, limitations, usages, restrictions, settings or operating procedures.
Maintenance & Servicing

Over the course of the life cycle of the device, the device will need maintenance or repairs. During the acquisition process, hospitals assess how much impact the regular maintenance will have on the device’s ability to operate, and if service to patients will be interrupted or if it can be bridged during maintenance.
Hospitals will consider:
- Can the desired service provider maintain the device? This may be the manufacturer or a third party. Unless otherwise stipulated, the hospital will put the maintenance contract out to tender for an appropriate supplier.
- What are the proposed time frames? How strict are they / is there flexibility?
- Continuity of care: Can the device be repaired on site? Are there other options for the model when a device is being repaired or serviced? What is the availability of spare devices or parts?
- Can the service provider guarantee response times and maintenance time frames? Are the time frames appropriate and reasonable?
- Are any calibrations or replacements required between official maintenance? Are the instructions on user calibration clear?
- How long is service support guaranteed?
- What information is available from the manufacturer / within the manufacturer instructions? e.g. Maintenance schedules, preventative maintenance plans, troubleshooting guidance, repairs procedures, parts lists, spare parts and specialist repair tools?
Finalising the Acquisition & Awarding the Contract
One of the final steps of acquisition is the drafting of a full performance specification of the entire system will be drafted prior to acquisition – this is part of the tendering process.
In addition, “full” means full, and includes every single conceivable aspect of a device including items like batteries and charging procedures. You must be prepared to draft and provide information on everything related to the device.
Once the performance specification is received and the device has been confirmed as an acquisition, final terms and conditions will be drafted and will be accepted and signed by all parties concerned. Only at this point will the contract officially be awarded to the supplier/manufacturer. The acquisition will then proceed according to the agreed terms, and any user feedback will be put towards future acquisitions/contract extensions.
Resource Download / Contact Us

This page is a brief overview of this resource topic, if you have found this information useful we strongly encourage you to download the full PDF resource.
You can download the main "Managing Medical Devices: Into the NHS" resource here: Managing Medical Devices: Into the NHS (PDF) and the supplementary resource that has the information unique to In Vitro Diagnostic devices and Point of Care Testing devices here: Managing Medical Devices: In vitro diagnostics (PDF)
You can also view the guidance we based this resource on here: Medical Devices: Guidance for Organisations (PDF), Management of In Vitro Diagnostic Devices (PDF), Management and Use of IVD and POCT devices (PDF).
We've also been provided with the Imperial College NHS Trust's local "Fixed Asset Policy & Procedure". Please note that this is due to be reviewed in December 2025, so some details may be subject to change. You can read the current version here: Imperial NHS Trust - Fixed Asset Policy & Procedure (PDF)
If you would like to contact us, or if there is a resource you would like to see, reach out to us using this form!
