Electrochemical interfaces are inherently complex and involve dynamic interactions between solid surfaces, reaction intermediates, solvated ions and solvent molecules. We fabricate well-defined interfaces, which provide a powerful approach to uncover the fundamental mechanisms that govern electrochemical reactions. We integrate this with complementary spectroscopic techniques, including operando X-ray (absorption, emission, photoemission, surface diffraction), vibrational (surface-enhanced infrared, Raman) and optical spectroscopy, to enable the direct observation of reaction intermediates, changes in oxidation state, adsorbate configurations, adsorbate-adsorbate interactions and dynamic surface reconstruction under realistic electrochemical conditions. This multimodal approach yields a holistic understanding of catalyst behaviour at the solid–liquid interface, bridging the gap between atomic-scale structure and performance. Such insights are critical for establishing structure–activity relationships and accelerating the rational design of next-generation electrocatalysts for sustainable energy and chemical technologies.
Electrochemical Reactions
- Proton Exchange Membrane Water Electrolysis
- Alkaline Water Electrolysis
- Low-grade water electrolysis
- Electrosynthesis
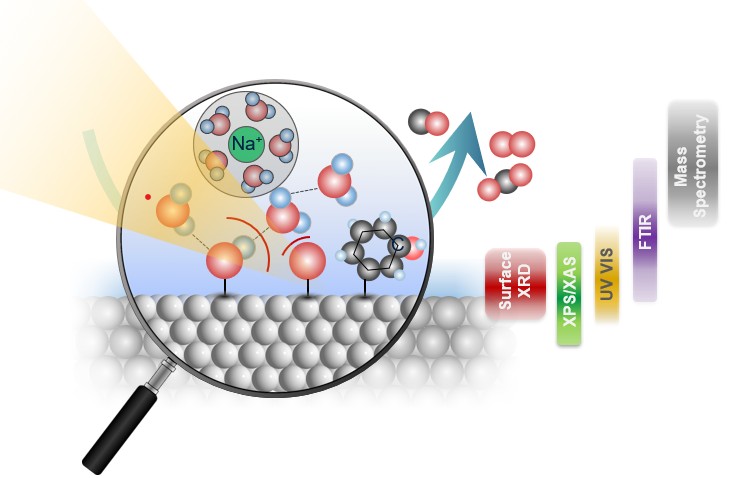
 Hydrogen is expected to play a central role in achieving net-zero emissions, offering a clean and flexible energy carrier that produces no carbon dioxide at the point of use. Among the technologies for green hydrogen production, proton exchange membrane (PEM) water electrolysers are particularly promising due to their high efficiency and compatibility with variable renewable electricity sources such as wind and solar. However, a major challenge facing PEM electrolysers is their reliance on iridium, one of the scarcest and most expensive elements, as the oxygen evolution reaction electrocatalyst in acidic environments. Our research focuses on studying iridium oxide to reduce (and possibly eliminate) its use while maintaining performance and durability.
Hydrogen is expected to play a central role in achieving net-zero emissions, offering a clean and flexible energy carrier that produces no carbon dioxide at the point of use. Among the technologies for green hydrogen production, proton exchange membrane (PEM) water electrolysers are particularly promising due to their high efficiency and compatibility with variable renewable electricity sources such as wind and solar. However, a major challenge facing PEM electrolysers is their reliance on iridium, one of the scarcest and most expensive elements, as the oxygen evolution reaction electrocatalyst in acidic environments. Our research focuses on studying iridium oxide to reduce (and possibly eliminate) its use while maintaining performance and durability.
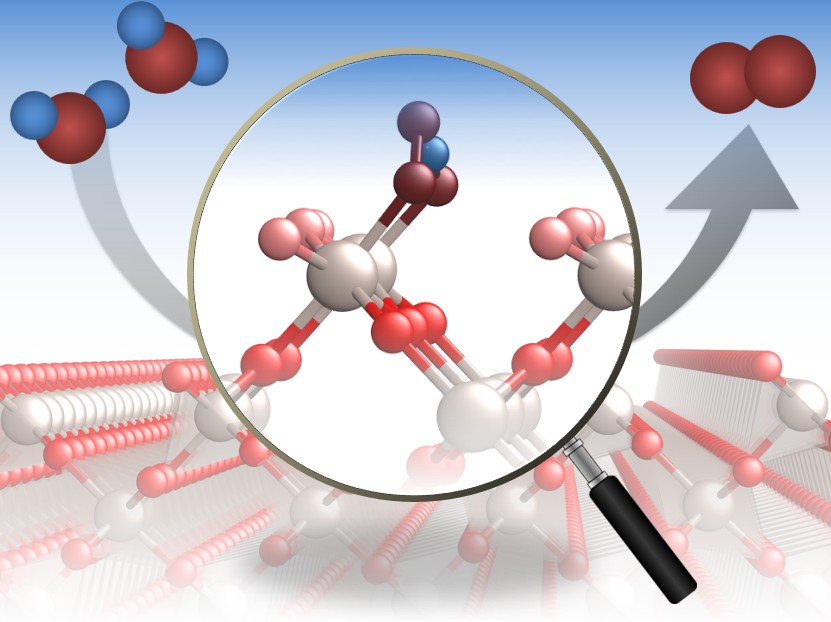
 Although alkaline water electrolysers have been the workhorse of green hydrogen production for decades, under operating conditions, it is unknown whether commercial metallic catalysts exist in the oxide, hydroxide, metallic or hydride phase. Catalysts also deactivate after only a few hours of operation, with no consensus about the cause. We use operando spectroscopy and advanced microscopy techniques to understand the active phase of catalysts under reaction conditions and deactivation pathways.
Although alkaline water electrolysers have been the workhorse of green hydrogen production for decades, under operating conditions, it is unknown whether commercial metallic catalysts exist in the oxide, hydroxide, metallic or hydride phase. Catalysts also deactivate after only a few hours of operation, with no consensus about the cause. We use operando spectroscopy and advanced microscopy techniques to understand the active phase of catalysts under reaction conditions and deactivation pathways.
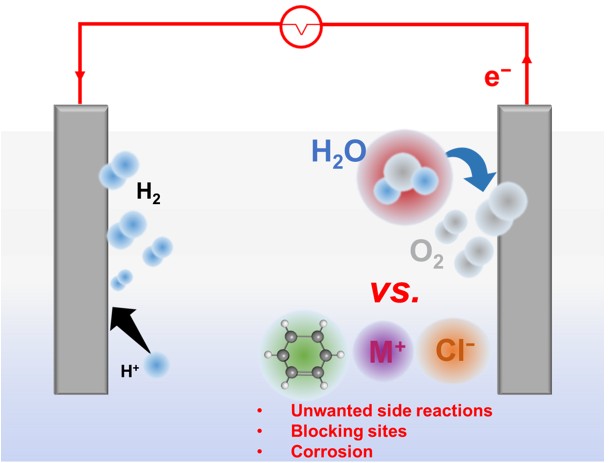 Our research is focused on investigating the use of impure water sources - such as seawater and wastewater for green hydrogen production. We are focused on developing low-cost, Earth-abundant and resilient catalysts for the oxygen evolution reaction at the anode that exhibit high activity, selectivity and stability under these challenging conditions. A central objective is to minimize unwanted side reactions, like chlorine evolution, while retaining high oxygen evolution activity.
Our research is focused on investigating the use of impure water sources - such as seawater and wastewater for green hydrogen production. We are focused on developing low-cost, Earth-abundant and resilient catalysts for the oxygen evolution reaction at the anode that exhibit high activity, selectivity and stability under these challenging conditions. A central objective is to minimize unwanted side reactions, like chlorine evolution, while retaining high oxygen evolution activity.
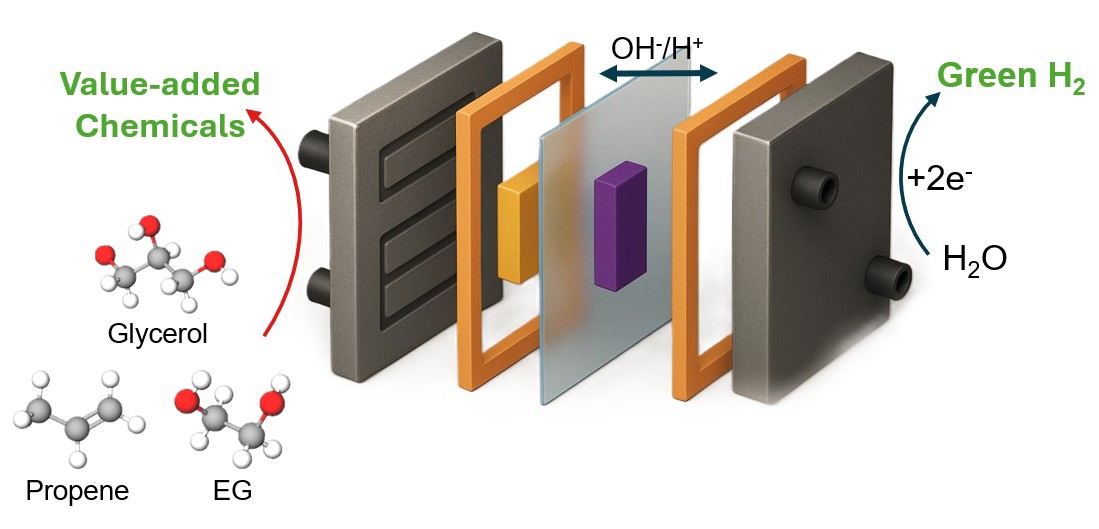 Coupling hydrogen production at the cathode with partial hydrocarbon oxidation at the anode to produce high-value chemicals not only circumvents these challenges associated with water electrolysis but also simultaneously enables the electrification of the chemical industry. The upscaling of these technologies relies on developing catalysts that can promote the selective and partial oxidation of hydrocarbons to commodity chemicals such as alcohols, aldehydes and acids and prevent its complete oxidation to carbon dioxide. Our research focuses on designing catalysts for selective oxidation of a number of organic substrates to value-added chemicals.
Coupling hydrogen production at the cathode with partial hydrocarbon oxidation at the anode to produce high-value chemicals not only circumvents these challenges associated with water electrolysis but also simultaneously enables the electrification of the chemical industry. The upscaling of these technologies relies on developing catalysts that can promote the selective and partial oxidation of hydrocarbons to commodity chemicals such as alcohols, aldehydes and acids and prevent its complete oxidation to carbon dioxide. Our research focuses on designing catalysts for selective oxidation of a number of organic substrates to value-added chemicals.