Important terms
Colonoscopy – a camera test to look inside the bowel
FIT test – a poo sample is tested for microscopic amounts of blood
Polyp – small growth on the inside of the bowel that can sometimes develop into cancer
Surveillance – having regular tests to prevent the development of cancer
Overview
The FIT for Follow-Up study began in 2012 and included around 8,000 people. It studied whether a stool sample test called a FIT was a good alternative to a colonoscopy for people who previously had bowel polyps removed and needed further testing.
What is a FIT?
A Faecal Immunochemical Test (FIT) can find blood in poo that isn’t visible to the naked eye. Blood in poo is a result of bleeding in the bowel, which can be caused by bowel polyps or even bowel cancer. If a FIT is positive (suggesting the presence of polyps or cancer), the patient may need more tests, including a colonoscopy, to find the cause of the blood.
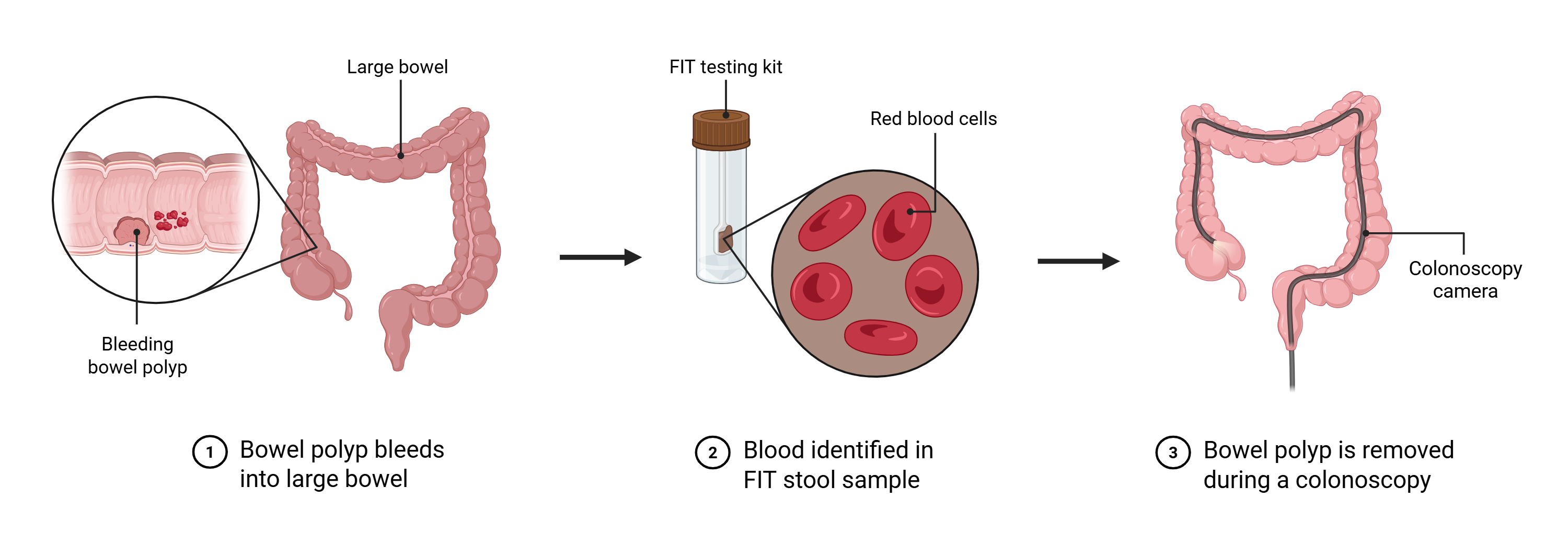
FIT method
The FIT can be sent to the person to be tested in the post, is simple to complete and can be done at home.
What is a colonoscopy?
A colonoscopy is a type of camera test. During a colonoscopy, a doctor passes a small camera on the end of a flexible tube through the bottom and into the bowel of a patient. The doctor looks at the inside of the bowel for anything unusual. Colonoscopies can either be used to diagnose problems or to provide treatment.
What is a polyp?
A polyp is a small growth on the inner surface of the bowel. They are usually found during a colonoscopy. Polyps are common and are not cancer but if left in the bowel they can sometimes develop into cancer. For this reason, they are usually removed during colonoscopies. 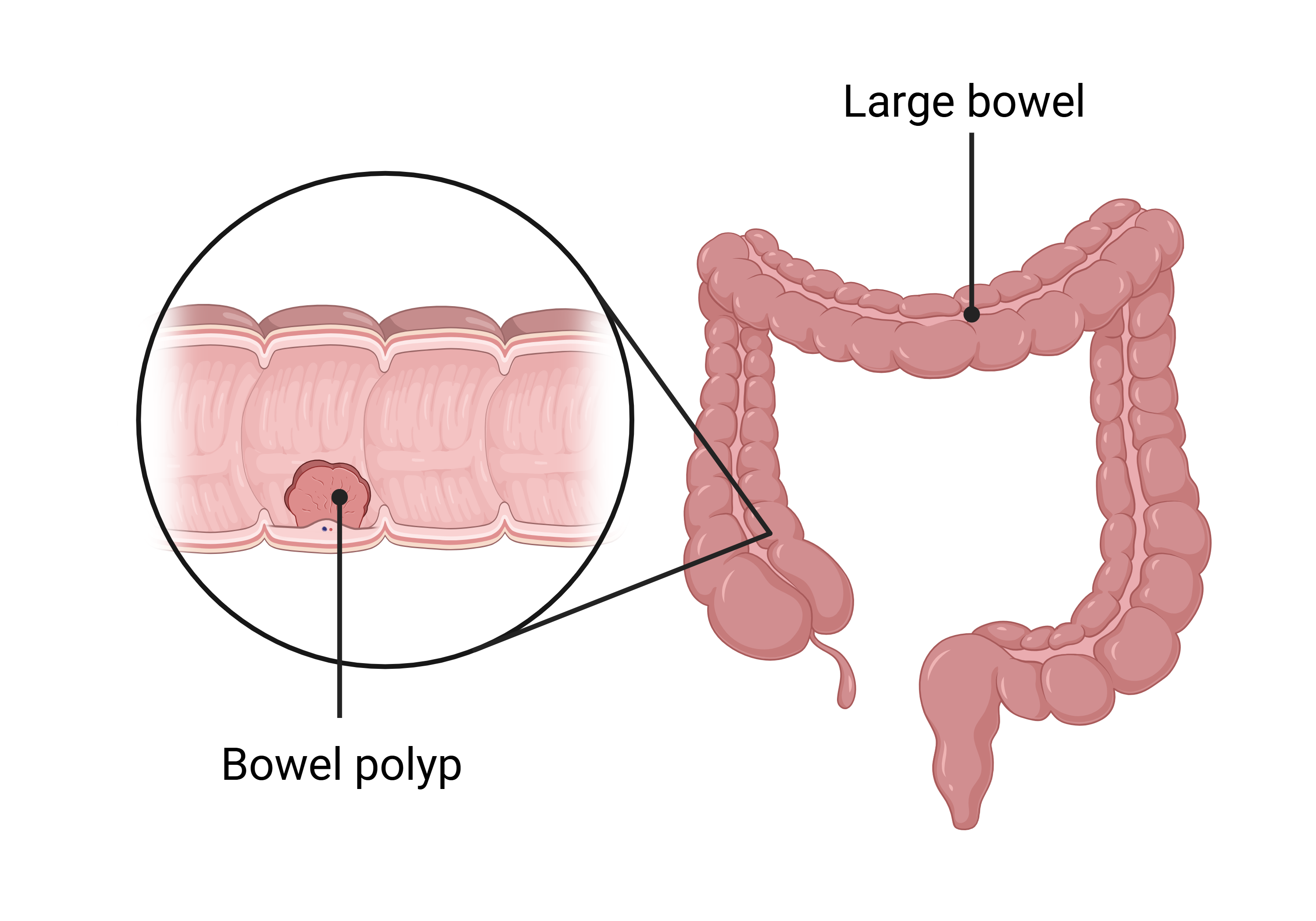
The type and number of polyps found will affect a person’s risk of developing bowel cancer in the future.
Why was this study needed?
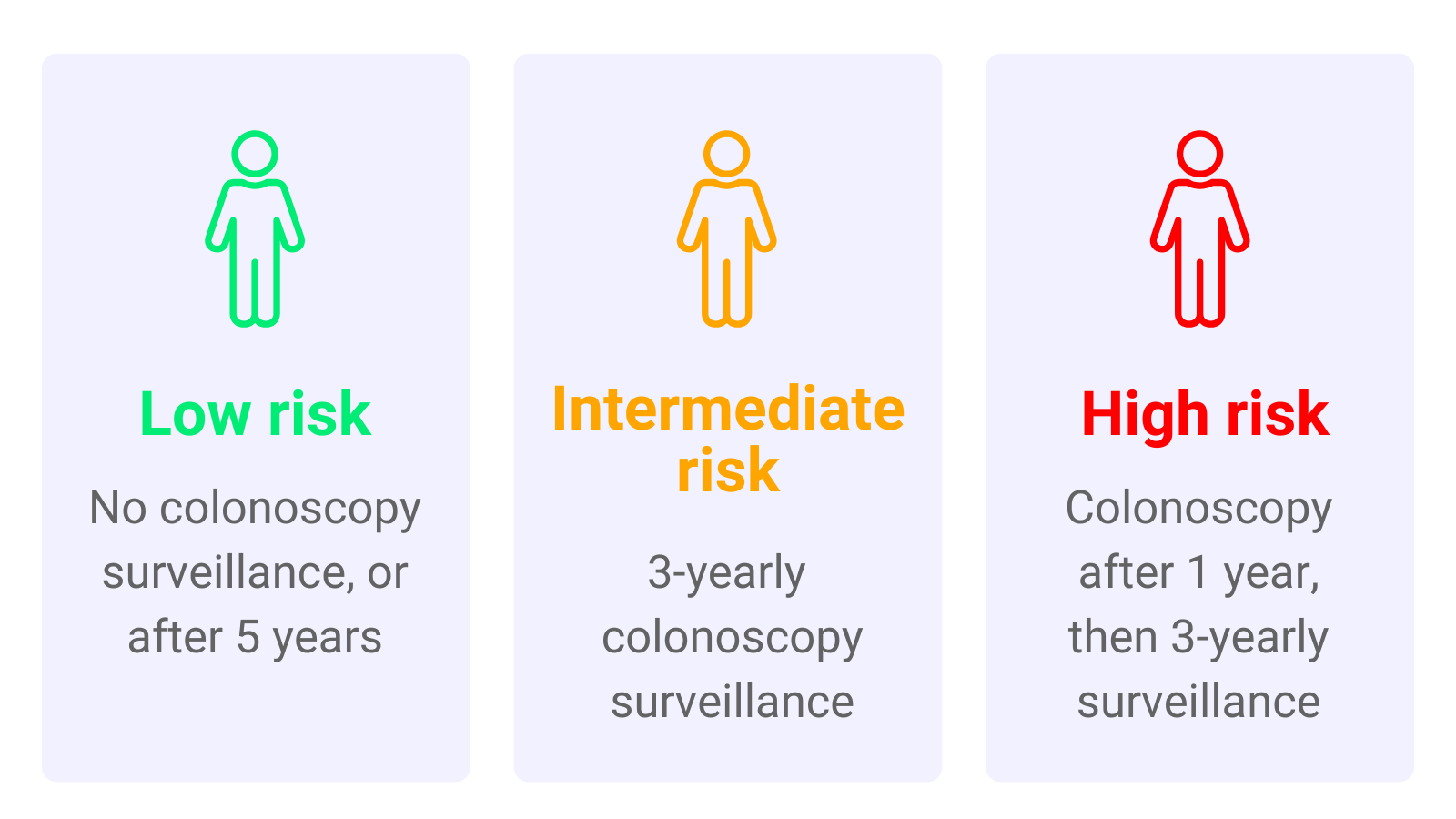
At the time of this study people found to have polyps were split into groups depending on their risk of developing bowel cancer. These groups were:
- Low-risk group.
- Intermediate-risk group.
- High-risk group.
Further colonoscopy tests were recommended for some groups to protect them from bowel cancer. This testing is known as ‘surveillance’. Low-risk people were recommended no surveillance or surveillance 5 years after their polyp was found. People in the intermediate-risk group were recommended to have surveillance after 3 years. People in the high-risk group were recommended surveillance after 1 year, and then 3-yearly.
2002 Post-Polypectomy Surveillance Guidelines
Colonoscopy after 3 years for people with intermediate-risk polyps helped to protect them against bowel cancer. However, colonoscopies are expensive, can be uncomfortable, and carry a small risk of serious complications. So, doctors were looking for other tests that could replace or reduce the number of colonoscopies.
A possible alternative was the FIT. Patients who needed surveillance could have yearly FITs instead of a 3-yearly colonoscopy. Anyone with over a certain amount of blood in their stool would have a positive FIT result, and would then go on to have a colonoscopy to find any further polyps or bowel cancer.
This would reduce the number of people having this uncomfortable test, reduce strain on colonoscopy services and save the NHS money. However, doctors needed evidence that the FIT was good at finding the right people for colonoscopy before it could be introduced to the NHS.
What was the aim of this study?
We wanted to find out if having yearly FITs was a safe and effective alternative to having a 3-yearly colonoscopy. We also looked at whether people were happy to do the test, and if it was a good use of NHS resources.
How was the study carried out?
People were invited to take part in the study via the NHS Bowel Cancer Screening Programme. We invited people who:
- Were aged between 60-72 years of age.
- Had taken part in the NHS Bowel Cancer Screening Programme.
- Had intermediate-risk polyps on their colonoscopy and were waiting for their 3-yearly colonoscopy.
We invited around 8,000 people and nearly 6,000 agreed to take part. We offered them a FIT to complete each year while they waited for their colonoscopy. We set the amount of blood found in the poo, to decide if a test was positive or not, at 40 micrograms of blood for every 1 gram of poo, or 40 µg/g.
If a patient had a positive result (showed a risk for cancer) on any of the three FITs, they were sent for their colonoscopy immediately. If they were negative on all FITs, they had their scheduled colonoscopy 3 years after their polyps were removed. 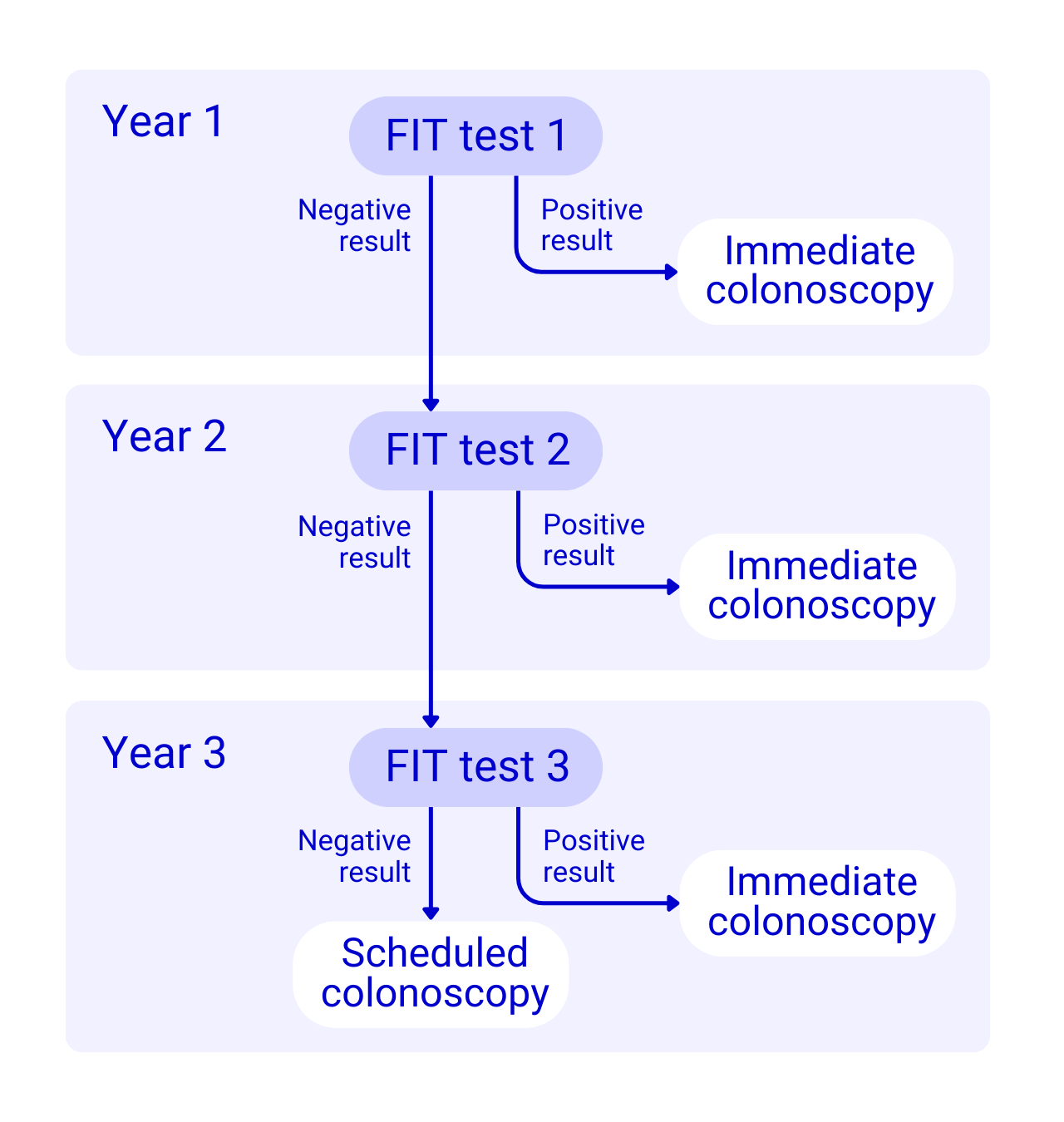
We compared the results of the FIT with the results of the colonoscopies. This showed us if the FIT was able to find all the people with polyps or bowel cancer. It was important to find out if people with polyps or bowel cancer were being missed by the FIT. We also wanted to know if the test was incorrectly flagging people for colonoscopies who didn’t need them.
When and where did the study run?
The FIT for Follow-Up study ran in England. It began in February 2012 and recruitment was completed by December 2013.
The collection of data from the people taking part was completed in 2016. We also received further cancer diagnosis data from Public Health England in 2017.
We will continue to study this data until 2028.
Who are funding FIT for Follow-Up?
FIT for Follow-Up was first funded by the National Institute for Health Research and Care Health Technology Assessment (NIHR-HTA) programme. This government programme funds research that studies how effective NHS-provided tests are. The study is currently funded by Cancer Research UK.
What are the results of the study?
The study demonstrated that an annual FIT could identify 72 of every 100 bowel cancers and 57 of every 100 patients with advanced polyps if repeated over 3 years. These results were published in the journal Gut.
.png)
Annual FITs were considerably cheaper than colonoscopy. Participants reported that the FIT was easy to use and provided reassurance. However, some were concerned that the FIT would not be as effective as a colonoscopy.
The results of the FIT for Follow-Up study were also published in the NIHR-HTA Journals Library.