Important terms
Cost-effective – the balance of affordability and effectiveness (benefit to patients) of a test
Colonoscopy – a camera test to look inside the bowel
Polyp or adenoma – a small growth inside the bowel that can sometimes develop into cancer
Polypectomy – removal of a polyp, usually during a colonoscopy
Surveillance – having regular tests to prevent the development of cancer
Overview
The Intermediate Adenomas (IA) study began in 2006. This then became a larger study in 2017 called the All Adenomas (AA) study, which is still ongoing.
The IA study reviewed the 2002 national guideline for monitoring people with bowel ‘polyps’ who are at intermediate (medium) risk of developing bowel cancer. We wanted to find out if the guideline was giving the correct advice to prevent cancer for this group, or if it needed to be updated.
In 2017, we expanded the IA study to include further groups of people with bowel polyps. We included people with polyps that had a low risk of developing into bowel cancer, and those with polyps that had a high risk of developing into cancer. This became known as the All Adenomas study.
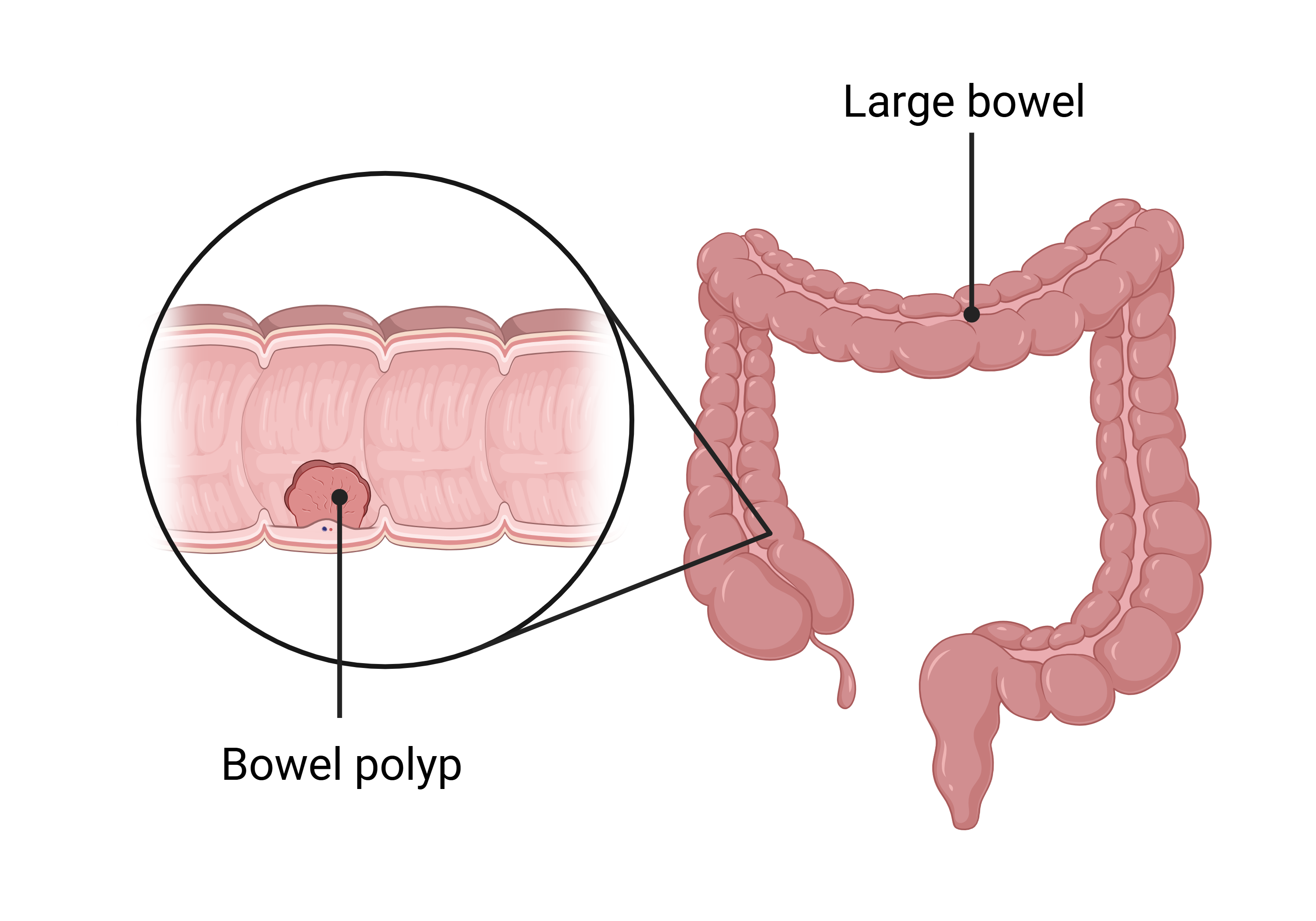
What is a bowel polyp?
A polyp is a small growth inside the bowel. They are usually found during a colonoscopy. Polyps are common and are not cancer but if left in the bowel, they can sometimes develop into cancer. For this reason, they are usually removed during colonoscopies. The process of removing a polyp is called a ‘polypectomy’.
 What is a colonoscopy?
What is a colonoscopy?
A colonoscopy is a type of camera test. During a colonoscopy, a doctor passes a small camera on the end of a flexible tube through the bottom and into the bowel of a patient. The doctor looks at the inside of the bowel for anything unusual. Colonoscopies can either be used to diagnose problems, or to provide treatment.
What was the 2002 UK post-polypectomy surveillance guideline?
National guidelines tell doctors the best way to look after patients. This ensures that all patients are getting the same, and best, treatment.
The 2002 UK post-polypectomy guideline described how doctors should monitor patients who have had a polyp removed. This monitoring is known as ‘surveillance’. It usually involves another colonoscopy. Surveillance helps to prevent bowel cancer in patients with previous polyps.
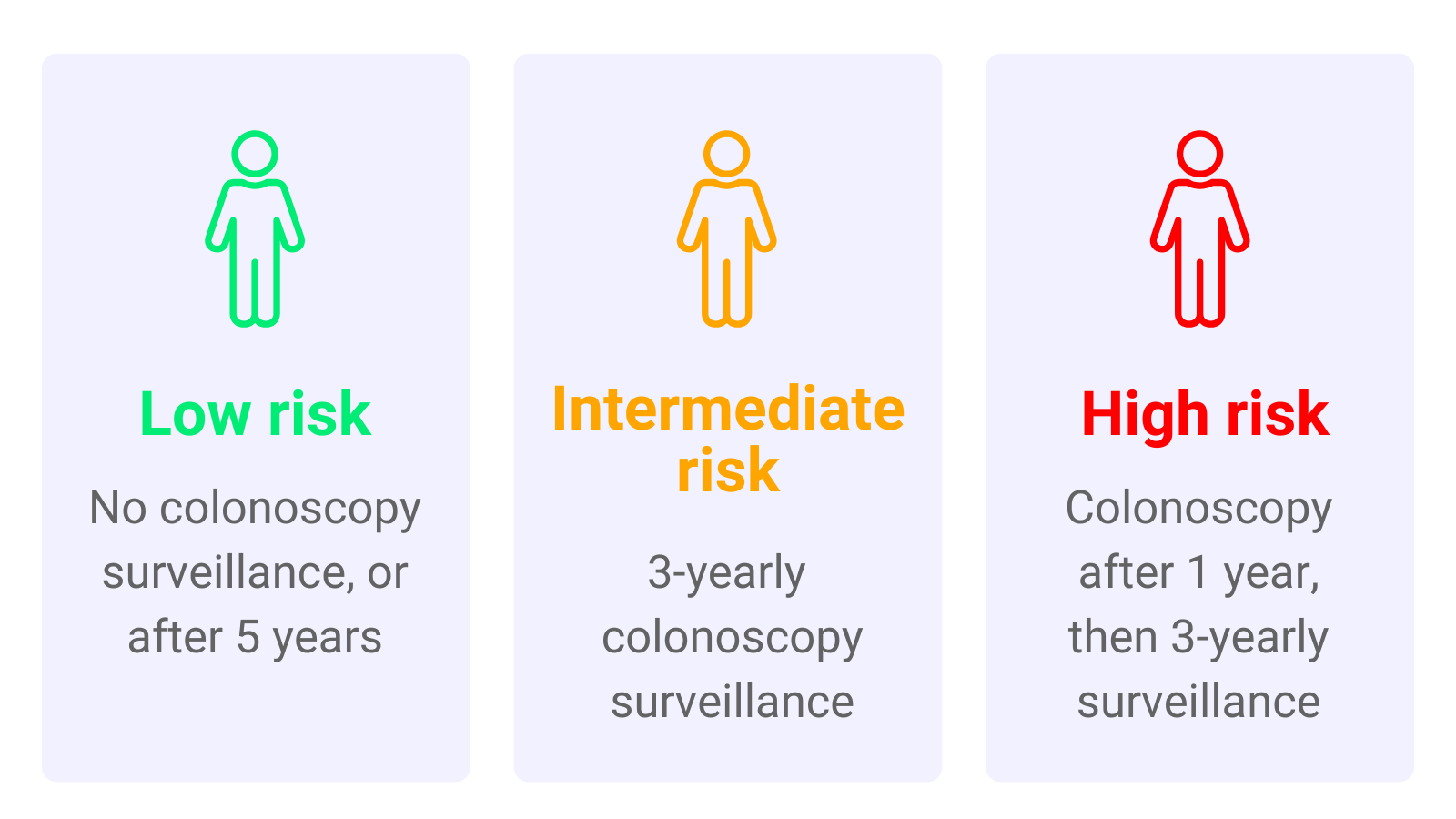
Depending on the type and number of polyps found, a person’s risk of developing bowel cancer will vary. Patients with polyps are split into groups depending on how likely they are to develop bowel cancer in the future. These groups were:
- Low-risk group
- Intermediate-risk group
- High-risk group
Under the 2002 guidelines, low-risk patients didn’t usually need surveillance; therefore they were recommended no surveillance or surveillance 5 years after their polyp was removed. Intermediate-risk patients were recommended surveillance after 3 years. High-risk patients were recommended surveillance one year after polyp removal, and then again after 3 years.
The 2002 Post-Polypectomy Surveillance Guidelines
Why were the studies needed?
The 2002 guideline needed to be updated as it had been several years since it was first written. It was important that only those who really needed a colonoscopy were having one. This is because a colonoscopy is expensive, uncomfortable and carries a small risk of serious complications.
Updating national guidelines requires strong scientific evidence from research studies. Our aim was to provide this evidence with the IA and AA studies.
What were the aims of both studies?
The purpose of the IA study was to discover if all intermediate-risk patients benefited from a 3-yearly colonoscopy. We thought it was likely that some of the patients were benefiting, while others didn’t need 3-yearly tests.
The AA study expanded on IA to see if the guidelines for the low-risk and high-risk patients were correct as well. We also investigated how much value for money to the NHS the 2002 guidelines were.
Our aim was to update the 2002 guidelines using the evidence from our studies.
How were the studies carried out?
Both studies looked at medical records from the past. The IA and AA are what are called observational, retrospective cohort studies.
- Retrospective – This means that the study used medical records from the past, rather than following people in the present into the future. A benefit of this is that researchers don’t have to wait several decades to collect results so the study can be completed faster. For IA, all the colonoscopies and cancer diagnoses had already occurred by the time the study data was collected.
- Observational – This means that we did not interact with the people included in the study, we just looked at their medical records.
- Cohort study – This means we looked at everyone who had polyps found during a colonoscopy at a number of hospitals. We didn’t pick and choose which patients to include.
We reviewed the medical records of over 30,000 people that had an adenoma, a common type of polyp, found during colonoscopy between 1972 and 2010 at one of 17 hospitals (see below).
We recorded the type and number of polyps found, the length of time between colonoscopies, and whether people developed bowel cancer or not. Using this data, we could calculate if surveillance colonoscopies were necessary for each of the different risk groups of patients.
When and where did the study take place?
Data was collected from 17 participating hospitals across the UK (see Protecting your Data for full list of hospitals). The study began in 2006 and is ongoing until 2028.
Who is funding IA and AA?
The IA study was funded by the National Institute for Health Technology Assessment Programme (NIHR-HTA). This is a government programme that funds research about how effective NHS tests are. The study was also funded by the Cancer Research UK Bobby Moore Fund.
The AA study was also funded by the NIHR-HTA and is currently funded by Cancer Research UK.
What are the results of the studies?
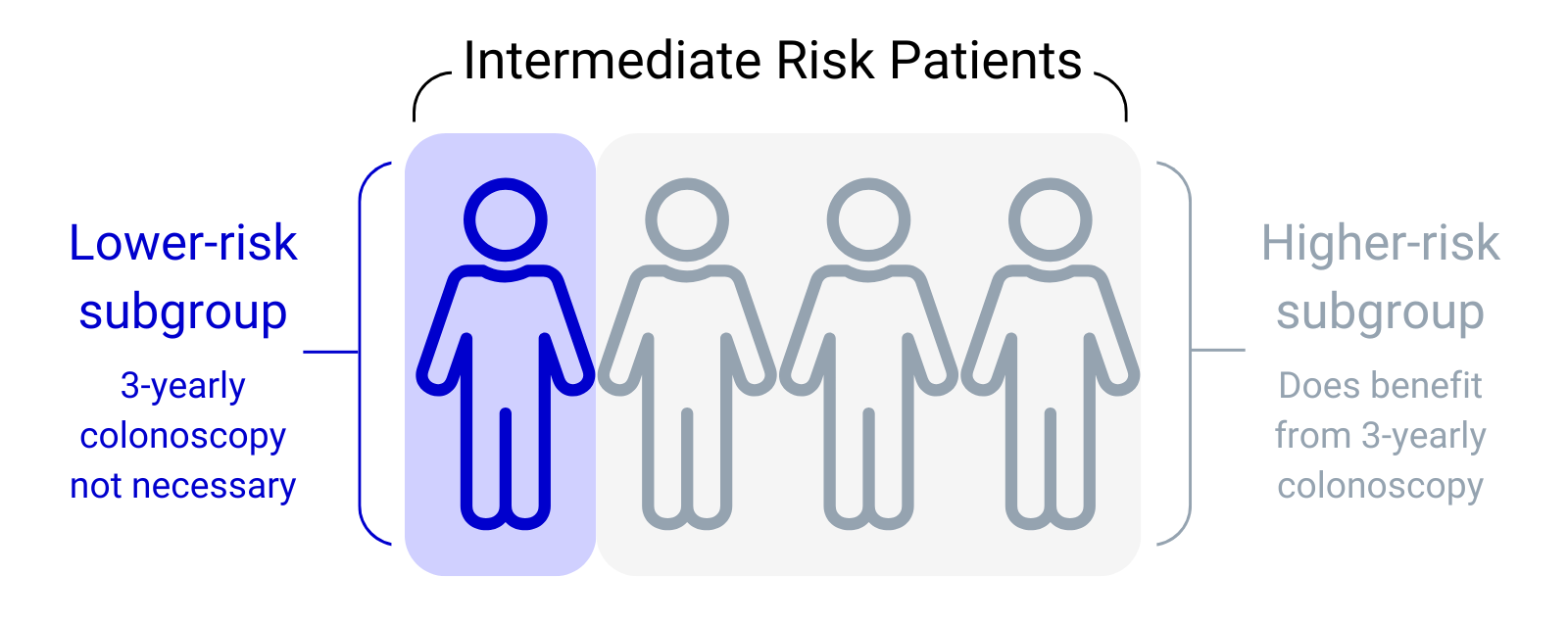
The IA study showed that most patients with intermediate-risk polyps did benefit from a surveillance colonoscopy. This matched the advice given in the 2002 guideline. However, the results also showed that around 25% of these patients had a much lower risk of bowel cancer than first thought. This suggested that surveillance colonoscopies may not be needed for this small, lower-risk subgroup of patients.
These findings were published in The Lancet Oncology and the NIHR-HTA Journals Library in 2017.
The AA study confirmed that patients in the low-risk group were very unlikely to develop bowel cancer and did not need any surveillance colonoscopies. We also found that the high-risk patients should have surveillance colonoscopies to protect them against bowel cancer. These findings matched the advice in the 2002 guideline.
The AA study also reviewed the previous intermediate-risk patient data from the IA study. We confirmed that there is a lower-risk subgroup within the intermediate-risk group and that this sub-group does not benefit from surveillance.
These findings were published in the journal Gut in 2020 and in the NIHR-HTA Journals Library in 2022.
What impact have these studies had?
Our findings show that some intermediate-risk patients don’t need the level of surveillance recommended by the 2002 guideline. Our work helped in the development of the updated 2020 UK post-polypectomy surveillance guidelines.
These updated guidelines are helping the NHS to save money and resources. They ensure that people with a high risk of bowel cancer are protected while keeping low-risk patients from having unnecessary tests.
Why are we doing further work with AA?
The updated 2020 guidelines put patients into only two groups of high and low risk for bowel cancer. The high-risk group are advised to have a surveillance colonoscopy after three years. The low-risk group are not advised to have surveillance. We applied these risk groups to the AA study data. The results confirmed that the newer guidelines are good at dividing patients into a group at higher risk of bowel cancer and a group at lower risk. These findings were published in the journal Gut in 2021.
We continue to analyse IA and AA data. We have identified more important research questions including:
- Determining when surveillance can safely stop in terms of age and number of colonoscopies.
- Examining the effect of surveillance on the risk of dying from bowel cancer.