Contact
2006, 2nd Floor, Institute of Reproductive and Developmental Biology, Hammersmith Campus
Lab/office contact number: +44 (0) 20 7594 2137
What we do
Our group aims to answer two questions:
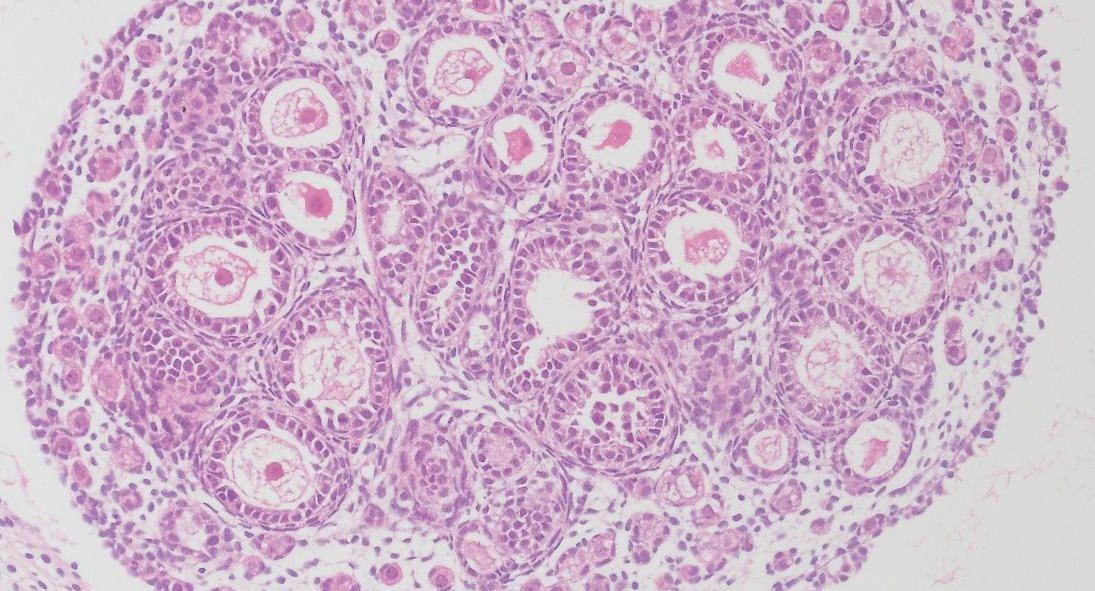
- What regulates the assembly and early development of ovarian follicles in health and disease?
- Can we identify molecular determinants of oocyte quality to estimate embryo development potential?
We investigate the genetic and epigenetic regulation of gonadal development and function, and its impact on gametes. To understand these processes, we integrate and apply omics approaches including chromatin proteomics, single cell sequencing and ATAC-sequencing, to cells derived from transgenic animal models as well as to samples donated by patients undergoing fertility treatments.
We focus particularly on understanding how the development of the ovarian follicles is controlled within the somatic cell lineages, in health and disease such as polycystic ovarian syndrome (PCOS), and the impact that this process has on long-term female fertility and oocyte quality.
In additional studies with collaborators, we investigate the regulatory mechanisms important for somatic cell fate maintenance using mouse gonads as experimental model.
Why it is important
Global fertility rates are steadily declining. It is predicted this will produce a contracting population with fewer young people relative to older people before the end of the 21st century. Ovulatory disorders cause 40% of female infertility cases, with specific ovulatory conditions such as PCOS affecting 1 in 10 women worldwide. Advancing knowledge in folliculogenesis could therefore benefit many women. Our mission is to advance fundamental scientific knowledge in reproductive and developmental biology to improve reproductive health and fertility outcomes. Crucially, our research addresses an important gap in understanding oocyte quality determinants and folliculogenesis regulation in women.
On a fundamental science level, our research provides a model to understand nuclear functions (chromatin remodelling) and its links to a physiological process (gametogenesis).
How it can benefit patients
By identifying the key drivers of early folliculogenesis and by understanding their effects on oocyte quality, we could develop strategies to (i) improve diagnosis and treatment of reproductive disorders and infertility, and (ii) to assess oocyte quality, thus enhancing reproductive outcomes.
Summary of current research
- Identify epigenetic signatures of oocyte quality in human cumulus cells to predict embryo development and pregnancy outcomes, led by Dr Roberta Migale and Professor Stephen Franks (collaborators: Professor Aylin Hanyaloglu and Professor Robin Lovell-Badge).
- Investigating the role of USP7 in granulosa cell differentiation and primordial follicle formation in the ovary, led by Dr Roberta Migale.
- Regulation of gonadal cell fate maintenance, in collaboration with Dr Francis Poulat, Institute of Human Genetics, Montpellier.
- Epigenetic regulation of ovarian cell fate maintenance, Co-PI with Prof Patrick Western, Hudson Institute of Medical Research, Melbourne.
Information
Internal
- Professor Stephen Franks, Faculty of Medicine, Department of Metabolism, Digestion and Reproduction, Imperial
- Professor Aylin Hanyaloglu, Faculty of Medicine, Department of Metabolism, Digestion and Reproduction, Imperial
External
- Professor Robin Lovell-Badge, Francis Crick Institute
- Dr Helen O’Neill, UCL
- Dr Francis Poulat, Institute of Human Genetics, Montpellier
- Professor Patrick Western, Hudson Institute of Medical Research, Melbourne
- Migale, R.*, Neumann, M., Mitter, R., Rafiee, M. R., Wood, S., Olsen, J., & Lovell-Badge, R.* (2024). FOXL2 interaction with different binding partners regulates the dynamics of ovarian development. Science Advances, 10(12), *co-corresponding author.
- Gregoire, E. P., De Cian*, M. C., Migale, R.*, Perea-Gomez, A., Schaub, S., Bellido-Carreras, N., Stevant, I., Mayere, C., Neirijnck, Y., Loubat, A., Rivaud, P., Sopena, M. L., Lachambre, S., Linssen, M. M., Hohenstein, P., Lovell-Badge, R., Nef, S., Chalmel, F., Schedl, A., & Chaboissier, M. C. (2023). The -KTS splice variant of WT1 is essential for ovarian determination in mice. Science, 382(6670), 600-606. *co-author
- Walker, A. R., Larsen, C. B., Kundu, S., Stavrinidis, C., Kim, S. H., Inoue, A., Woodward, D. F., Lee, Y. S., Migale, R., MacIntyre, D. A., Terzidou, V., Fanelli, F., Khanjani, S., Bennett, P. R., & Hanyaloglu, A. C. (2022). Functional rewiring of G protein-coupled receptor signaling in human labor. Cell Reports, 40(10), 111318.
- Rossitto, M., Dejardin, S., Rands, C. M., Le Gras, S., Migale, R., Rafiee, M. R., Neirijnck, Y., Pruvost, A., Nguyen, A. L., Bossis, G., Cammas, F., Le Gallic, L., Wilhelm, D., Lovell-Badge, R., Boizet-Bonhoure, B., Nef, S., & Poulat, F. (2022). TRIM28-dependent SUMOylation protects the adult ovary from activation of the testicular pathway. Nature Communications, 13(1), 4412.
- Migale R, Neumann M, Lovell-Badge R. (2021), Long-Range Regulation of Key Sex Determination Genes. Sexual Devevelopment.15(5-6):360-380. doi: 10.1159/000519891.
- Eozenou, C., Gonen, N., Touzon, M. S., Jorgensen, A., Yatsenko, S. A., Fusee, L., Kamel, A. K., Gellen, B., Guercio, G., Singh, P., Witchel, S., Berman, A. J., Mainpal, R., Totonchi, M., Mohseni Meybodi, A., Askari, M., Merel-Chali, T., Bignon-Topalovic, J., Migale, R., (. . .), Bashamboo, A. (2020). Testis formation in XX individuals resulting from novel pathogenic variants in Wilms' tumor 1 (WT1) gene. Proc Natl Acad Sci U S A (PNAS), 117(24), 13680-13688.
- Migale, R., MacIntyre, D. A., Cacciatore, S., Lee, Y. S., Hagberg, H., Herbert, B. R., Johnson, M. R., Peebles, D., Waddington, S. N., & Bennett, P. R. (2016). Modeling hormonal and inflammatory contributions to preterm and term labor using uterine temporal transcriptomics. BMC Medicine, 14(1), 86.
- Migale, R., Herbert, B. R., Lee, Y. S., Sykes, L., Waddington, S. N., Peebles, D., Hagberg, H., Johnson, M. R., Bennett, P. R., & MacIntyre, D. A. (2015). Specific Lipopolysaccharide Serotypes Induce Differential Maternal and Neonatal Inflammatory Responses in a Murine Model of Preterm Labor. American Journal of Pathology, 185(9), 2390-2401.
- MacIntyre, D. A.*, Lee, Y. S.*, Migale, R., Herbert, B. R., Waddington, S. N., Peebles, D., Hagberg, H., Johnson, M. R., & Bennett, P. R. (2014). Activator protein 1 is a key terminal mediator of inflammation-induced preterm labor in mice. FASEB J, 28(5), 2358-2368. *co-author.
Our researchers
Dr Roberta Migale
/prod01/channel_3/media/images/people-list/Roberta-Migale.jpg)
Dr Roberta Migale
Principal Investigator