New trial to evaluate intestine implant to treat obesity related type 2 diabetes
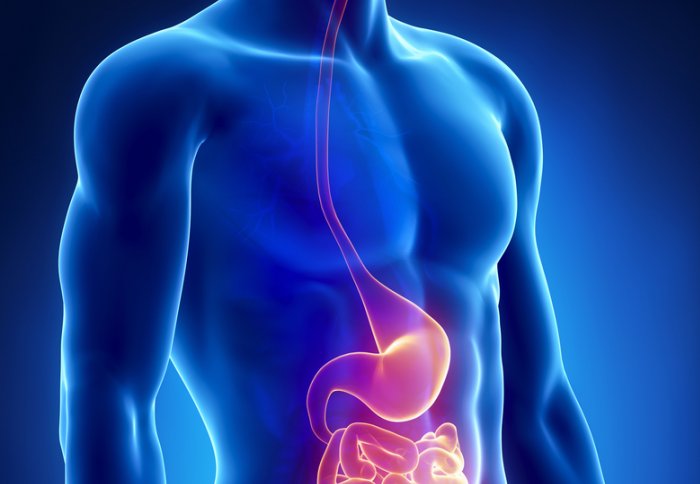
A new trial has been launched to investigate the effectiveness of a tube-like device inserted into the small intestine to treat type 2 diabetes.
The trial will compare the effects of the Endobarrier with standard medical care in the UK. By collecting detailed data on weight loss and diabetic symptoms, the research will investigate if the device could provide a viable and possibly superior approach to treating type 2 diabetes in people living with obesity.
The Endobarrier is a 60 cm tube-like device that is placed in the small intestine to create a thin layer between the food and the wall of the intestine. This ensures the food bypasses a section of upper intestine and does not make contact with the intestine, potentially altering the level of hunger and fullness.
Using a lower cost and less invasive alternative to bypass surgery could enable us to treat thousands more people living with type 2 diabetes every year
– Professor Julian Teare
Professor of Gastroenterology
The Endobarrier also prevents food mixing with bile and pancreatic juices, allowing the bile to be reabsorbed back into the bloodstream and potentially helping insulin work more effectively to lower blood sugar and alleviate diabetic symptoms. The device is delivered using an endoscope passed through the mouth, oesophagus and stomach, so it does not require invasive surgery.
Currently type 2 diabetes can be treated with duodenal bypass surgery, for which there is a long waiting time and a high cost, or by standard medical therapy that works with medication, diet and exercise. This trial will evaluate the viability of the device as an alternative to the standard medical care in the NHS and investigate the details of how it takes effect.
While previous clinical trials and commercial experience suggest that the EndoBarrier is a safe and effective treatment option for type 2 diabetes, results from this study should provide definitive evidence to help guide treatment decisions.
Professor Julian Teare from the Department of Surgery and Cancer, Imperial College London, who is leading the study, said: “Type 2 diabetes affects millions of people in the United Kingdom and many of these people have been unsuccessful at managing their diabetes with their current treatment regimens. One of the great advantages of the EndoBarrier is that it does not rely on surgery or medication to achieve its effect. Using a lower cost and less invasive alternative to bypass surgery could enable us to treat thousands more people living with type 2 diabetes every year.”

Endobarrier device
The trial will take place at Imperial College London and University Hospital Southampton. It is aiming to recruit 160 participants (80 from each site) who are aged 18-65 years, living with type 2 diabetes and with a BMI between 30 and 50. Participants will be randomly allocated to either have the Endobarrier or to receive standard medical therapy.
“This is the first time that the Endobarrier has been trialled at this scale,” said Christina Prechtl, Clinical Trials Manager at the School of Public Health, Imperial College London, who is in charge of running the trial. “The results will provide valuable information on its efficacy and viability as a treatment, and also insight into how it actually takes effect which will help us optimise its impact in the future. Participants will benefit from close monitoring of their condition and regular contact with the research team. We will be investigating a wide range of measures such as metabolism, brain activity, sensitivity to insulin, food preference and the quality of life of participants. Our aim is to thoroughly analyse how this device can be used most effectively within the health system to help people suffering with type 2 diabetes.”
Developed by GI Dynamics Inc., the EndoBarrier is the first endoscopically delivered device approved for the treatment of type 2 diabetes and obesity by lowering blood sugar and weight at the same time. Participants will be recruited to the trial for 24 months.
This project is supported by the Efficacy and Mechanism Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership.
The views and opinions expressed therein are those of the author and do not necessarily reflect those of the NIHR, MRC, NHS or the Department of Health.
For more information on the trial please see http://www1.imperial.ac.uk/biosurgerysurgicaltechnology/clinical_trials_outcomes/bariatricsurgery/endobarrier/
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Franca Davenport
Communications and Public Affairs