Innovation by evolution: bringing new chemistry to life

The Institute for Molecular Science and Engineering (IMSE) hosted Professor Frances Arnold to deliver an inspiring Highlight Seminar this week.
Frances Arnold is the Linus Pauling Professor of Chemical Engineering, Bioengineering and Biochemistry, as well as Director of the Donna and Benjamin M. Rosen Bioengineering Center at Caltech.
Power of a polymath
With a diverse educational and research background – degrees in mechanical and aerospace engineering and in chemical engineering, and postdoctoral experience in chemistry departments – Professor Arnold’s experience is an excellent example of the polymathy IMSE promotes.
Indeed, the Institute admits students with undergraduate degrees in a variety of subjects for their collaborative and industry-focused Master’s of Research in Molecular Science and Engineering programme. Moreover, during her talk, Professor Arnold described how her research incorporates most – if not all – of the disciplines, processes and scales that are merged in the molecular science and engineering research framework.
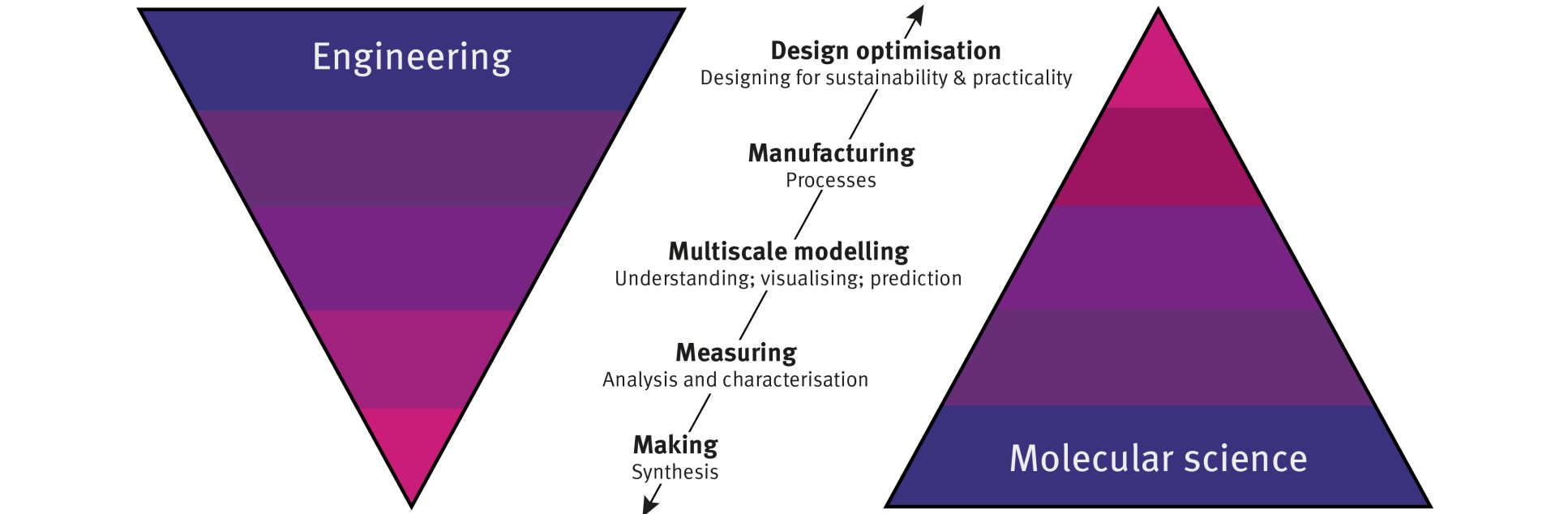
Inspired by nature
We’re trying to go beyond where both biology and chemistry have explored. Professor Frances Arnold Caltech
Professor Arnold started her career at the beginning of the ‘DNA revolution’ and was inspired by the efficiency of the natural world, specifically biological systems – well-organised machines capable of extracting energy and materials from the environment and converting them for self-replication and function.
With scientists’ ability to untangle the code of life, Professor Arnold’s research has been guided by the desire to program biological molecular machines for particular everyday applications. Technology advances have been tremendous – such that it is now possible to read, write and edit DNA sequences, but researchers still don’t understand DNA sufficiently to know how to compose it from scratch.
The problem of building something that is not fully understood is not novel. Engineers do it all the time – the process of ‘trial and error’. What’s more, biology has evolved in exactly this way, i.e., through a simple algorithm of mutation and natural selection.
Directed evolution: simple engineering
‘Directed evolution’ – based on the process of natural selection – is a standard optimisation strategy in modern protein engineering. This method is used to engineer industrial enzymes, e.g., those used in laundry detergents.
Despite the simple progress of directed evolution (and natural selection), innovative products can still be realised from naturally occurring enzymes that have never previously evolved for a particular function. This is because enzymes exhibit catalytic ‘promiscuity’. Random mutations are usually selected when they serve particular beneficial functions. Other – seemingly useless – mutations, however, can lie dormant for several generations until new conditions emerge and new functions become relevant.
Natural innovation
As an example of nature’s innovation, Professor Arnold described the case of atrazine – a widely used herbicide that was originally thought to be non-biodegradable. Indeed, its use was banned in the EU in 2003 when levels of the chemical in groundwater were found to be unsafe.
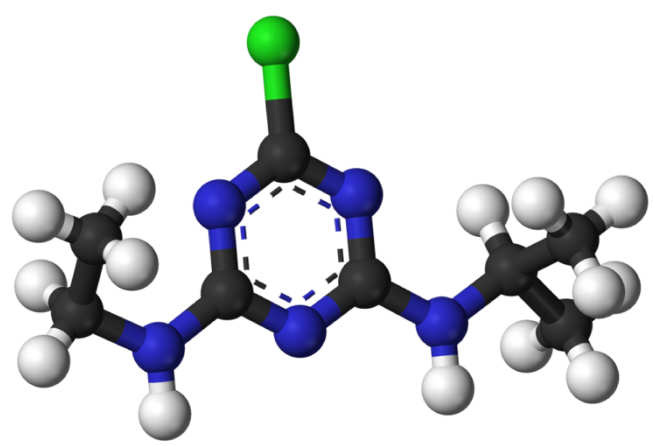
A set of discovered organisms, however, have evolved – by optimising their enzymes – to degrade man-made atrazine and create a new source of nitrogen to sustain themselves. “This native promiscuity of enzymes can be used by ‘enzyme engineers’ to create new, useful functions”, explained Professor Arnold.
Cutting-edge research
In some of her most recent work, Professor Arnold’s group have been exploring using directed evolution and optimisation techniques to create these new enzyme functions. For example, in work published in Science, her group reported the use of enzymes to catalyse the formation of carbon–silicon bonds. Although these two elements are some of the most abundant on Earth, such bonds do not occur naturally within biological systems.
Professor Arnold’s group finds that ‘hemeproteins’ can catalyse the formation of carbon–silicon compounds under physiological conditions – both in vitro and in vivo. Via directed evolution, they were able to achieve bond formation rates that were 15 times faster than state-of-the-art synthetic catalysts, and their route thus presents an environmentally friendly and efficient alternative to the standard process (used, for instance, in the pharmaceutical industry).
Upcoming IMSE seminars
The two remaining IMSE Highlight Seminars of this year’s series will take place in June.
Professor Philip Withers’s (University of Manchester) talk – Completing the picture: correlative multimodal information across length and timescales – will take place on Thursday 7 June.
Professor Sophia Haussener (EPFL) will visit on Thursday 21 June and deliver a talk titled Modelling, experimentation and scaling of solar fuel processing devices.
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Dr Shoshana Z Weider
Faculty of Engineering