Structure-activity relationships for therapeutics of neglected tropical disease
Comprehensive structure and activity data for novel inhibitors of Leishmania N-myristoyltransferase
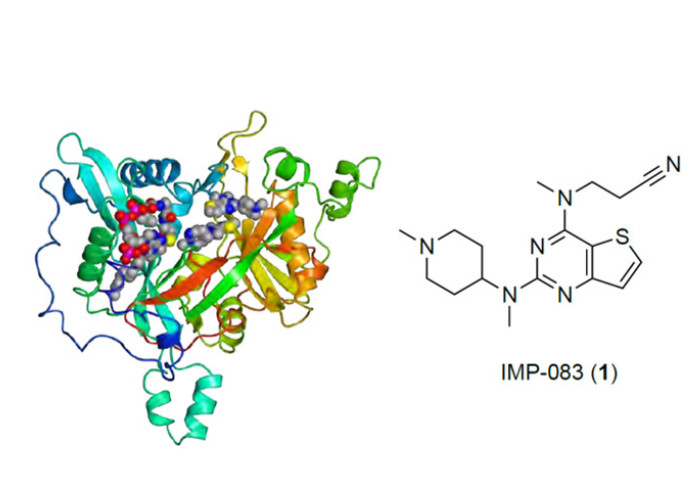
Tate group members (and alumni) collaborated with the York Biomedicial Research Institute, University of York, to produce a study in the Journal of Medicinal Chemistry on inhibitors of the enzyme N-myristoyltransferase (NMT) in the parasite Leishmania which causes the tropical disease Leishmaniasis.
Leishmania are protozoan parasites with various species which can cause different clinical symptoms, with up to 15 million infections across the world. The Imperial College London and University of York teams identified critical pathways in the parasites that could be targeted by therapeutics and built on the previously identified compounds that inhibit Leishmania NMT. NMT transfers fatty acid chains to protein molecules, and some of these modification targets are critical for parasite function.
This research delved into the structure-activity relationship of the compounds in detail, using x-ray crystal structure analysis and in-cell assays, ultimately improving the inhibitor selectivity for Leishmania over human NMTs. Additionally, the study also identified the cell-active inhibitors for the species Leishmania donovani.
This research was supported by The Wellcome Trust and the Engineering and Physical Sciences Research Council.
Article supporters
Article text (excluding photos or graphics) © Imperial College London.
Photos and graphics subject to third party copyright used with permission or © Imperial College London.
Reporter
Edward Bartlett
Department of Chemistry